Electrochemistry seems a little intimidating at first, but we explain some of the concepts here with videos, gifs and explanations and show you how it’s relevant with examples from everyday life. Expand anything you want to know more about. The downloadable worksheets contain real-world examples or give you ways to remember the key concepts. We are expanding this page, to provide more electrochemistry explanations, videos and resources, so check back and feel free to email us with any requests.
Our electrochemistry explanations
Understand the Potential of this area of science then break it down with Electrolysis before tearing it up with Corrosion and bringing it all back together with Electrodeposition and feel like a charged Battery by the end. Along the way, we have downloadable worksheets to give some theory or examples. Think you’ve missed an electrochemistry explanation sheet or real-life examples? A menu of them is on the left, for quick downloading.Introduction
Chemical reactions are all about electrons moving around. These reactions are spontaneous (they can happen whether we want them to or not, like rusting) or driven (where we need to provide a push to get them to happen).
Why is electrochemistry important?
Who cares if copper is less reactive than aluminium? What does it matter?
Electrochemistry is already a big (and growing) part of our lives. Here’s a few questions to think about…
-
Anyone got a battery on them?
- What about in your phone or watch?
- What about a pacemaker or a fire alarm system
- What do you think we would do without batteries?
-
Is anyone else glad that airplanes don’t get rusty?
- Lots of metals (like nickel, titanium, ruthenium) are used in electroplating. But why do metals corrode in the first place?
-
It’s good to have some hydrogen with you on your space travels. Why?
- Because using hydrogen in a fuel cell means you can have electricity and clean water.
- It’s why hydrogen fuel cells were used in the moon landings.
- Where else would fuel cells be useful?
-
What about sensors?
- Compare an electrochemical sensors, like the ones used for blood sugar for people with diabetes, to the tests you do for chemicals in the lab.
What would it be like if someone had to carry test tubes and chemicals to test their blood sugar? - Heart monitors rely on the bodies natural electrochemistry, which produces potentials, to measure tiny voltages on the skin. Some people are trying to incorporate these into normal clothes. Why?
- What else could we use sensors for?
- Compare an electrochemical sensors, like the ones used for blood sugar for people with diabetes, to the tests you do for chemicals in the lab.
-
Any ideas for the future?
- Perhaps we will be using electricity to coat materials in solar panel ink, which will dry and create energy.
- What about clothes or shoes that charge our phones by our movement?
- What about lower energy and faster processing, to reduce the effect of our phones on the planet?
Whether a reaction will happen spontaneously or needs to be driven will depend on the differences in energy for an electron at the beginning and end of the reaction. In electrochemistry, the energy it has is called it’s electrochemical potential. But first, let’s see how chemistry relates to electrochemistry in our first electrochemistry explanation sheet.
In this sheet, we see that electrochemistry takes many forms, from the table salt, to the organic chemistry we rely on for medications and polymers to rust and plating metals. It is everywhere in our everyday life. Electrochemical reactions are either “spontaneous” so they “want” to happen or driven, where they are pushed to happen using something else to get those electrons moving. In electrochemistry, this is often a battery or another source of electricity. But how do we know whether a reaction “wants” to happen? Well, in the next electrochemistry videos and explanations, we will introduce Electrochemical Potentials.
Electrochemical Potential
A high electrochemical potential is like a marble at the top of a marble run, ready to react. Some atoms “want” electrons more than others and they will pull them off materials that have them. Some atoms “want” to give up electrons, if they can. You might have seen the reactions of sodium or lithium before, which burst into flame when they react with water. They also react with oxygen and chlorine, the RSC have useful videos
here and resources for teachers from the RSC here.
What is the Reactivity Series?
This video introduces the reactivity series in the context of displacement reactions.
Electrochemical potential – GCSE
Before GCSE, you probably learnt about the reactivity series, where some metals will react faster than others. You have also learnt about the structure of atoms. These topics are related, some atoms ‘want’ to gain electrons to create a full outer shell so will pull electrons off another atom. Some atoms gain a full outer shell by losing their outer electrons, so ‘want’ to lose electrons. This sheet relates the reactivity series to electrochemical potential.
Electrochemical potential – A level
From the reactivity series, you are already familiar with electrode potentials. Instead of thinking about whether a metal “wants” to gain or lose an electron to fill an outer shell, two electrodes in solutions are used. One is the standard hydrogen electrode, the other is the electrode to be tested. So whether a metal “wants” an electron is tested by asking “Will it take an electron from hydrogen, or dump an electron onto a proton?”
How is Electrochemical Potential measured?
We have said that some metals prefer to lose electrons, some to gain them. But how do we know that? How are they measured?
The potential of one electrode cannot be measured, because it is measured as a voltage (a difference in potential) between two points. So if there was only one point, a difference couldn’t be measured. As well as the theoretical problem of measuring a potential of an electrode on it’s own, there is a practical problem. A voltmeter measures voltage by using a tiny current that won’t disturb the electrode too much. If there is no current, potential can’t be measured.
So a reference electrode must be used. This sheet describes other “zero” points we use. It also illustrates “potential difference”.
The Standard Hydrogen Electrode
The Standard Hydrogen Electrode is the “zero” point chosen for electrochemistry. The electrochemical process of a proton reduction with an electron into a hydrogen atom is a one electron process and the proton is small. When two hydrogen atoms form on the surface, they can form hydrogen gas and bubble away. It is described in the following sheet. The opposite process also occurs, where hydrogen can be oxidized into protons in solution. An equilibrium is reached and this is the potential of this electrode under set conditions. That means if another electrode is connected electrically with wires, a voltmeter and with a salt bride between the electrolytes, a comparison can be made to another electrode. In this way, a potential relative to this Standard Hydrogen Electrode is measured. If this is done under standard conditions (298K, 1 mol dm-3 and if gases are used, 1 atm) This value is by convention, given in potential difference in volts for the reduction reaction.
Oxidation happens with reduction
Electrons must go somewhere! All oxidation reactions must also be balanced by a reduction reaction. So if one atom has lost an electron, another one has gained it.
Redox chemistry
If you want to oxidise one atom of sodium, it loses one electron per atom. This sheet gives some examples of redox reactions.
Oxidation numbers
An oxidation number is a formal way of describing how many electrons a metal is missing. For instance Iron(III) means iron3+, Fe3+. A common form of Fe3+ is in a compound with oxygen. Oxygen, needing two electrons to fill its outer sphere, is usually has a 2- charge. You can’t balance one (3+) charge with a single (2-) charge. So iron (III) oxide exists as Fe2O3.
Oxidising and Reducing agents
An oxidizing agent is a chemical which oxidizes something else. As oxidation is loss, this means that the oxidizing agent gains the electron it takes from the thing that it oxidizes.
A reducing agent does the opposite, it reduces things. So it gets rid of it’s electrons by putting them on something else. The reducing agent is oxidised because it has lost electrons.
Redox titrations
Titrations at GCSE usually involve an acid and an alkaline. By knowing they will react in a certain proportion, it is possible to work out the concentration of one solution from the concentration of another. Redox titrations are similar, but instead of acid + alkali, the reactions involve the transfer of electrons. Sometimes this makes use of the colours available in transition metal compounds to monitor the reactions. This sheet summarizes the reaction between potassium permanganate and iron(II) compounds.
Examples of oxidation numbers in real life
With each breath in and out, you are using a change in oxidation number in a number of biological compounds that have metal cores. One of the most abundant is the haem structure in haemoglobin.
Vanadium has 3 electrons in it’s outer d sub-shell and another 2 electrons in it’s very outer s sub-shell. This means there are a number of oxidation states available to vanadium.
The transition in oxidation states can also be used to store energy. By using energy to oxidise one oxidation state and reduce another, then storing those complexes, energy is stored in the increased electrochemical potential of those complexes. This is how something called a “redox flow battery” and is beyond A-level, but is an interesting example of the uses of the numerous vanadium oxidation states.
Electrolysis
Electrolysis is made up of “electon” and “lysis”, which means “breakdown or dissolution”. The chemistry of a solution can change from solutions we are familiar with, like vinegar, but also can change because of current from a battery.
To see electrolysis in action, we are going to use an experiment with red cabbage indicator. All of these are normal household products and red cabbage indicator is pretty simple to make, download the worksheet and follow the instructions. Remember the hazards associated with household chemicals, this is a good opportunity to test your chemical hazard knowledge. Read the backs of the bottles and be careful!
Water Electrolysis – GCSE
Where the anode oxidises the water, it forms oxygen and at the cathode protons are reduced to hydrogen.
The second part of the video uses a 9V battery to achieve the same vivid colours in red cabbage indicator, which suggests an area of high pH and an area of low pH are forming.
Water Electrolysis – A level
How do you know if a reaction is spontaneous or non-spontaneous? We don’t see hydrogen and oxygen spontaneously forming on connected electrodes in a solution. But for some other reactions, this might be less obvious. From the electrochemical series, we can make an educated guess about whether the reduction for one material and the corresponding oxidation of another is likely to happen. There is also a way of calculating the Gibb’s Free Energy of a reaction from the electrode potentials. As with any other reaction, a negative Gibb’s free energy indicates a reaction is spontaneous. In an electrochemical reaction, the Gibb’s free energy is related to the standard potential of the reaction, the number of electrons involved and the Faraday constant, which converts between electrical units and chemical units.
Gibb’s Free Energy
Gibb’s Free Energy is a measure of how much energy a reaction or process needs to happen or releases when it happens. If the reaction has a negative Gibb’s energy, it is favourable, it can happen, it is spontaneous. The bonds that form (creating a more stable state for electrons) or the increased disorder/chaos created, or a balance of the two, favor the reaction.
If it has a positive Gibb’s Free Energy, energy must be supplied for it to happen. Otherwise it does not take place. These are non-spontaneous reactions.
The unintuitive part of this, is the second law of thermodynamics, which states the “entropy of a isolated system may never decrease over time”. That seems like a lot to learn. But this just means that things, with no external effects, tend towards mess, like a bedroom or the washing up. This is a law of thermodynamics, and not your fault. However, there is no law of thermodynamics that stops you learning with our electrochemical explanations.
This sheet introduces the calculations for the above reaction.
This one considers competition in this reaction.
Corrosion
Corrosion can be seen everywhere. See this sheet for the effect it has on ships.
Galvanisation is the process of coating one metal with another, which is higher in the reactivity series. A really common method, is galvanising steel or iron with zinc.
In this video, a household descaler solution, an acid, corrodes two zinc washers. But one washer is in contact with a platinum ring. Platinum is great at forming hydrogen gas from protons so acts as a catalyst for this reaction. The oxidation takes place on the zinc surface and the reduction on the platinum surface, they just need to be in electrical contact. Vinegar causes the same reaction but is a much weaker acid than descaler!
Electrodeposition
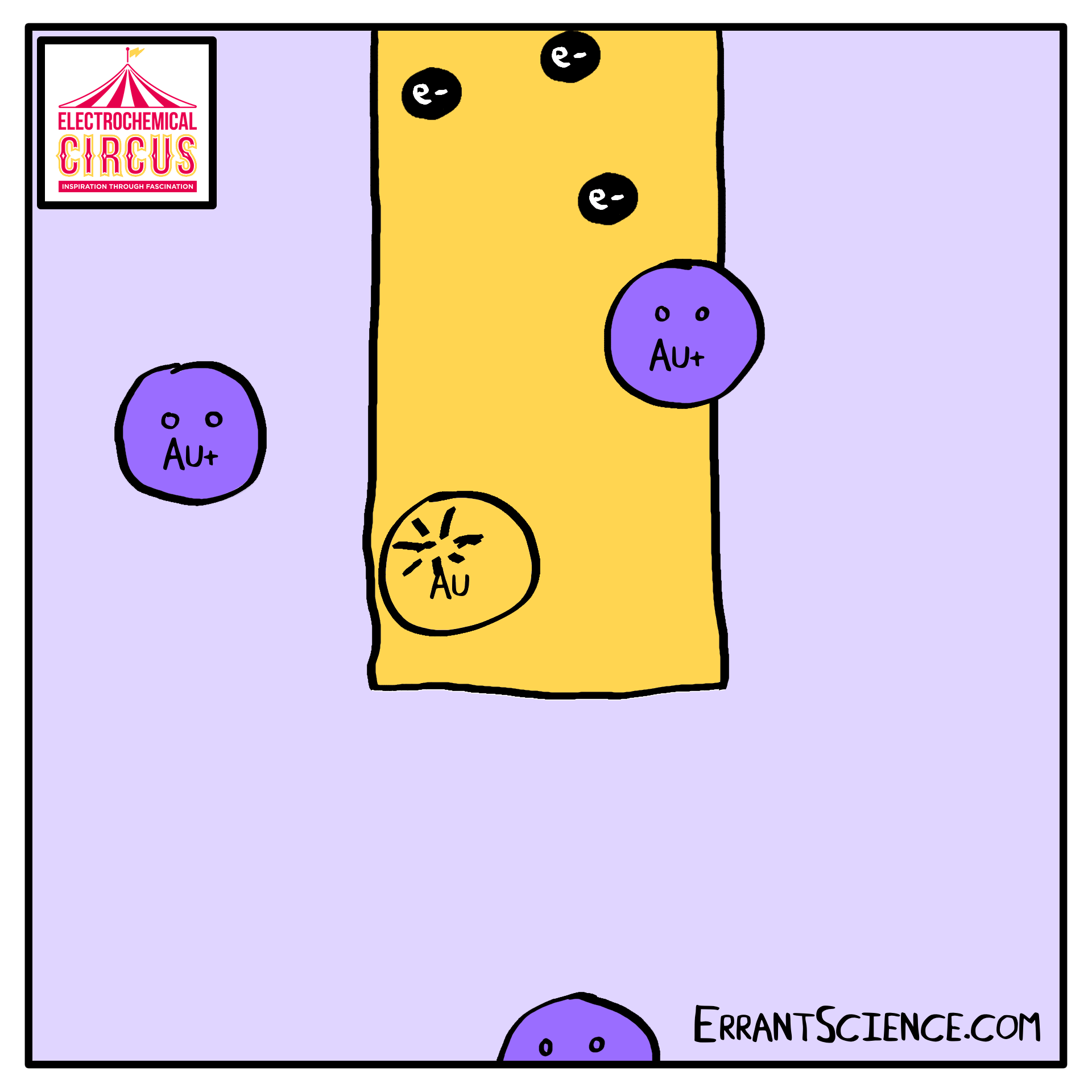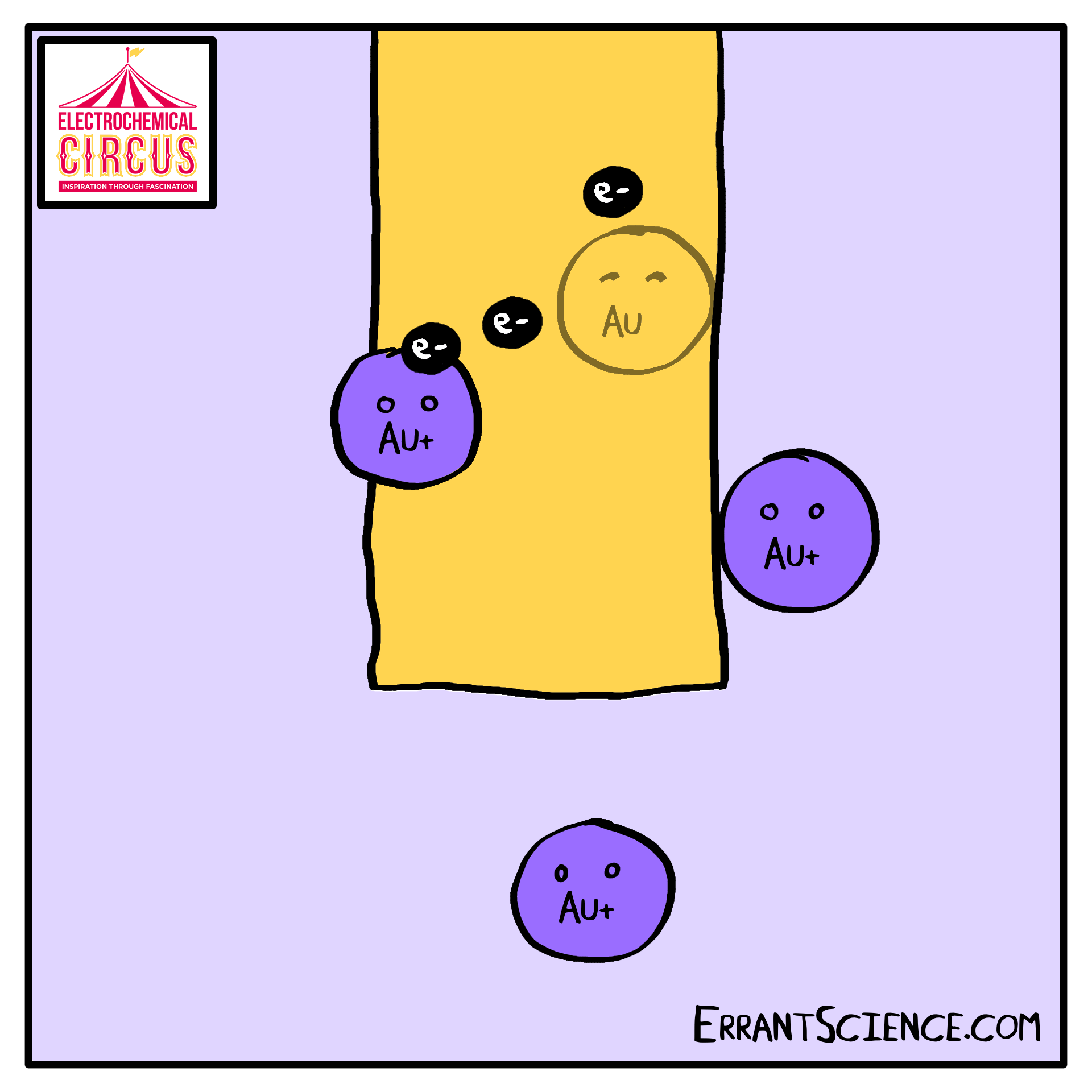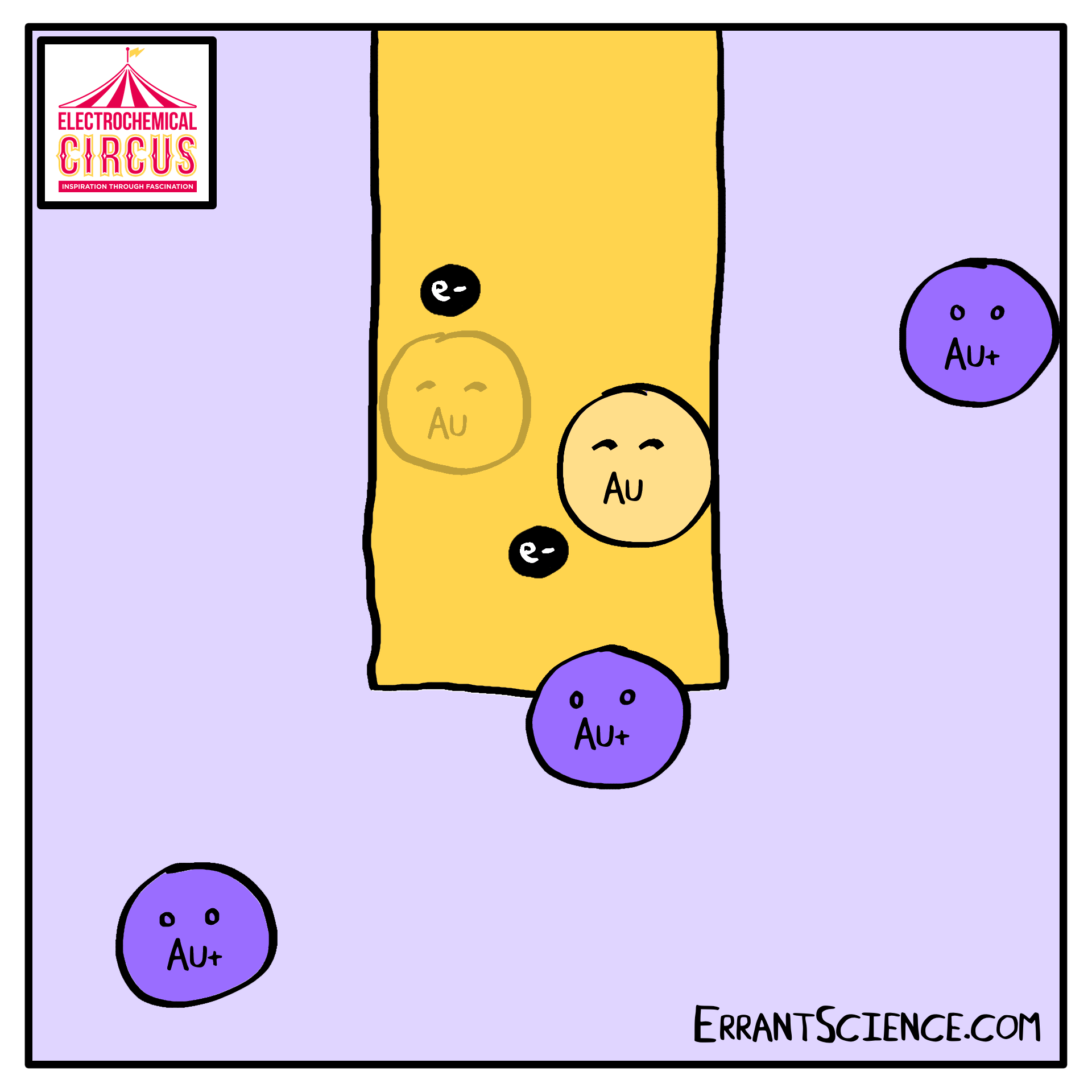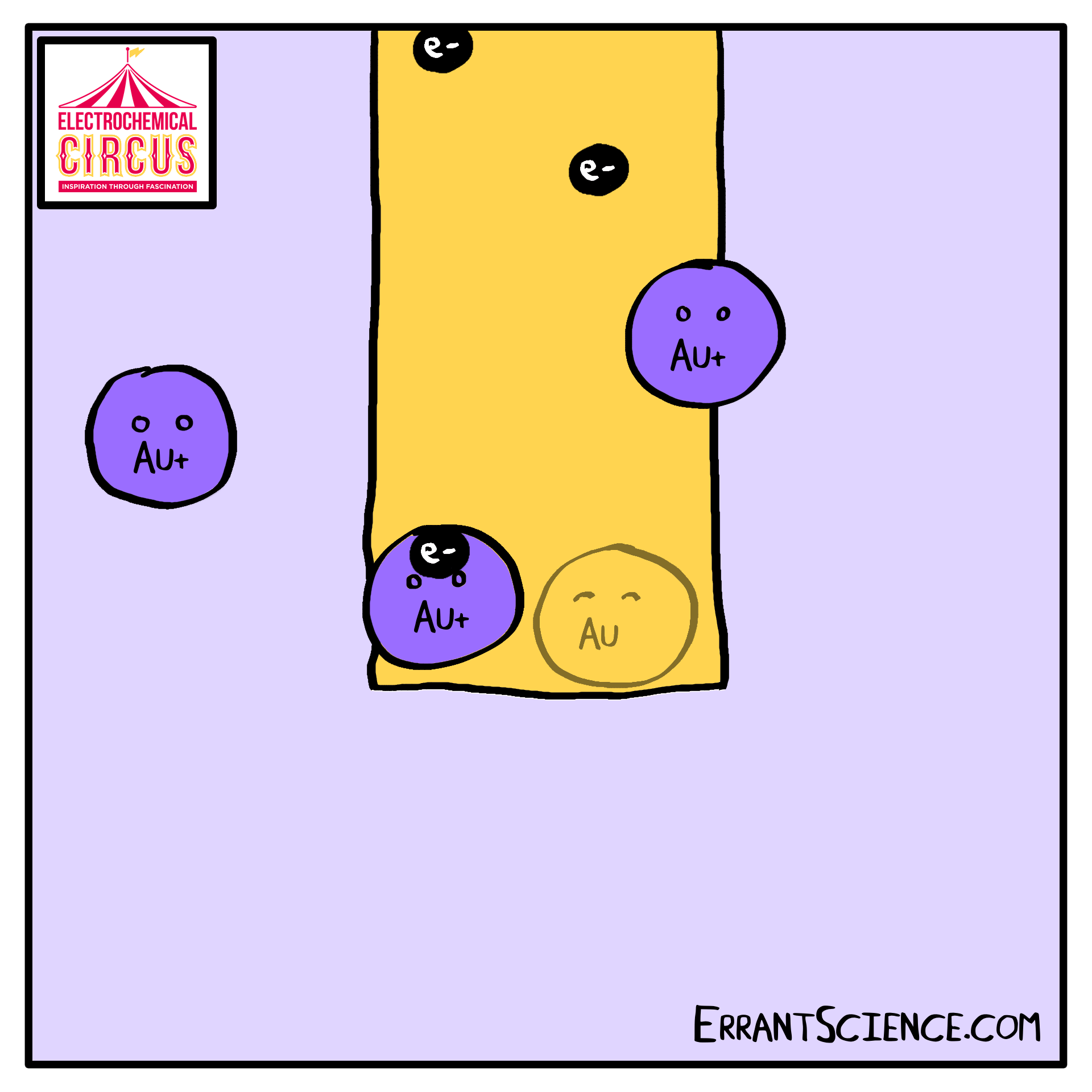
Electrodeposition is “electron” and “deposition”, where electrochemistry is used to deposit a layer onto something else. We use this as part of the Electrical Circus to create a layer of gold, using the grease from a fingerprint as a template. A solution of ions is reduced into metal.
One use of electrodeposition of metals is the production of computer devices. See our research page the research happening at Southampton on this. This worksheet is about the 100 million electrochemical devices you might have in your pocket right now. It is an electrochemistry explanation of how computer parts might be made in the future.
Batteries
Like the transistor, batteries have become so central to our technologies, we don’t even think about them any more. But batteries of all shapes and sized have a role to play in our future technologies and a transition to clean energy.
A battery uses a spontaneous reaction to drive electrons around a circuit. This is the opposite of the driven reactions. At each electrode, the reaction occurring there results in that electrode being at the potential of that reaction. There will be a difference in potential between the electrodes, the Potential Difference, measured in volts and providing the “push” for electrons travelling into one electrode, travelling around the circuit and back out of another.
If you put two reactive species in the same container, they will simply react. Instead, two “half-cells” are used and electronically separated from each other.
The Daniell Cell
The Daniell Cell is a great example of a spontaneous reaction that demonstrates the difference in potential between copper and zinc metals.
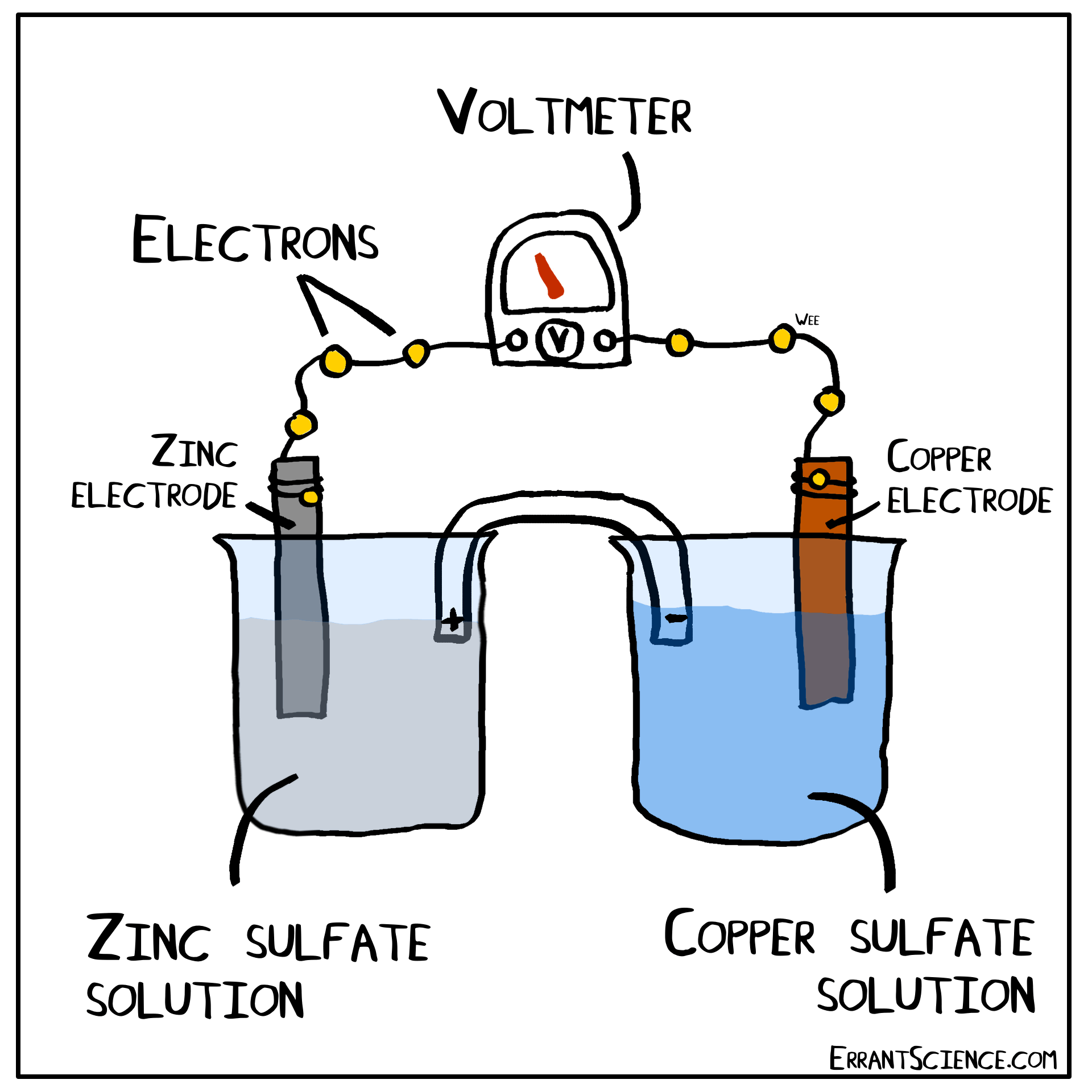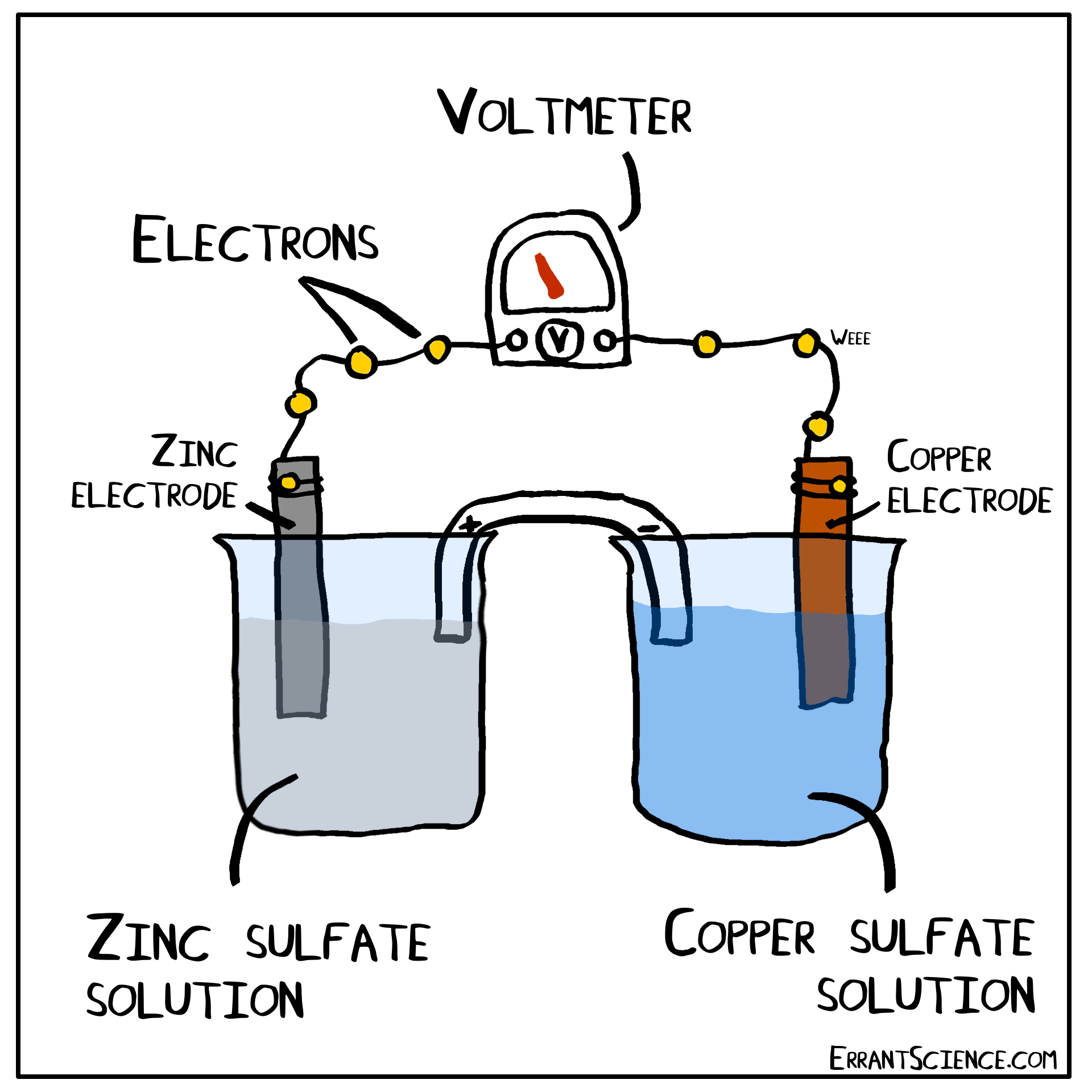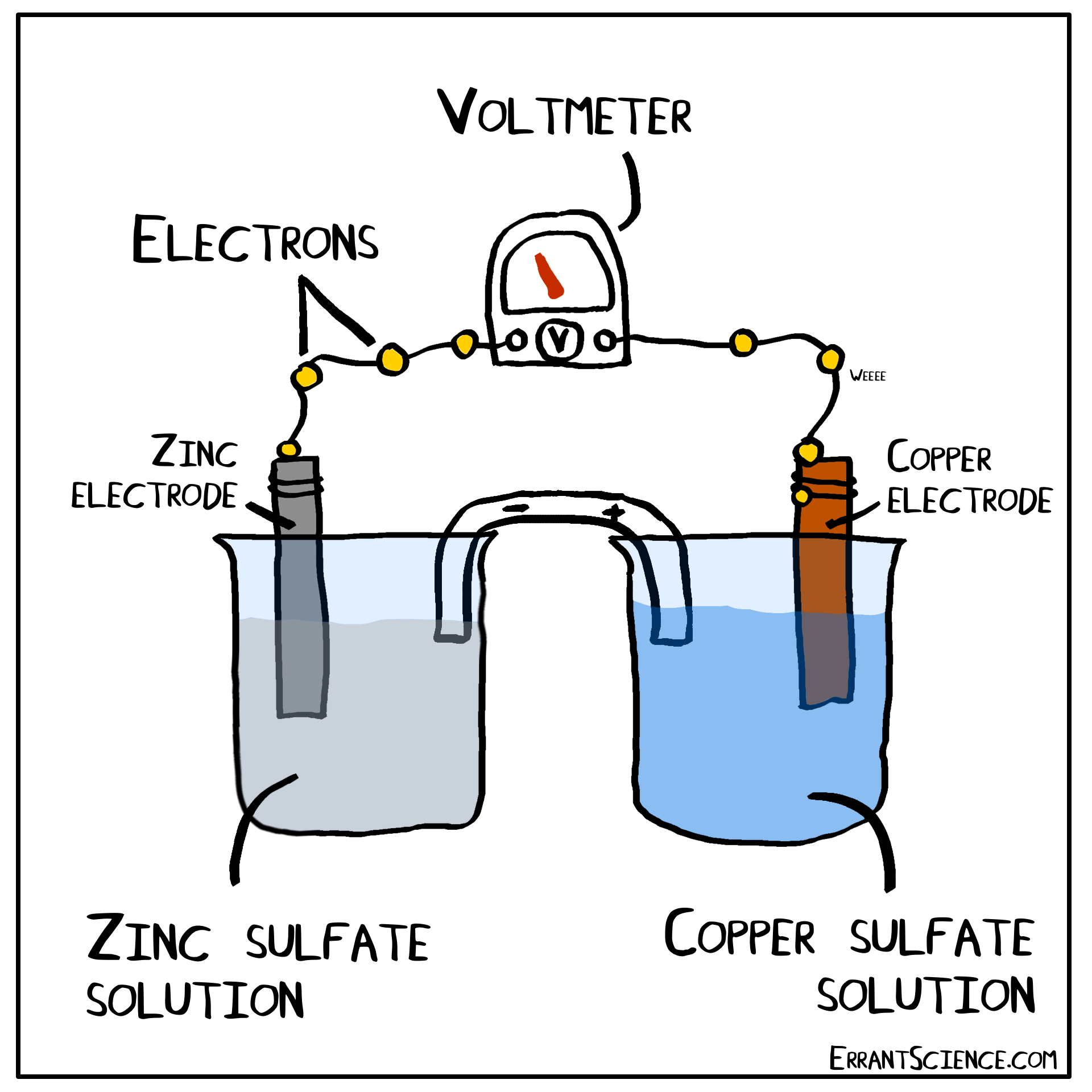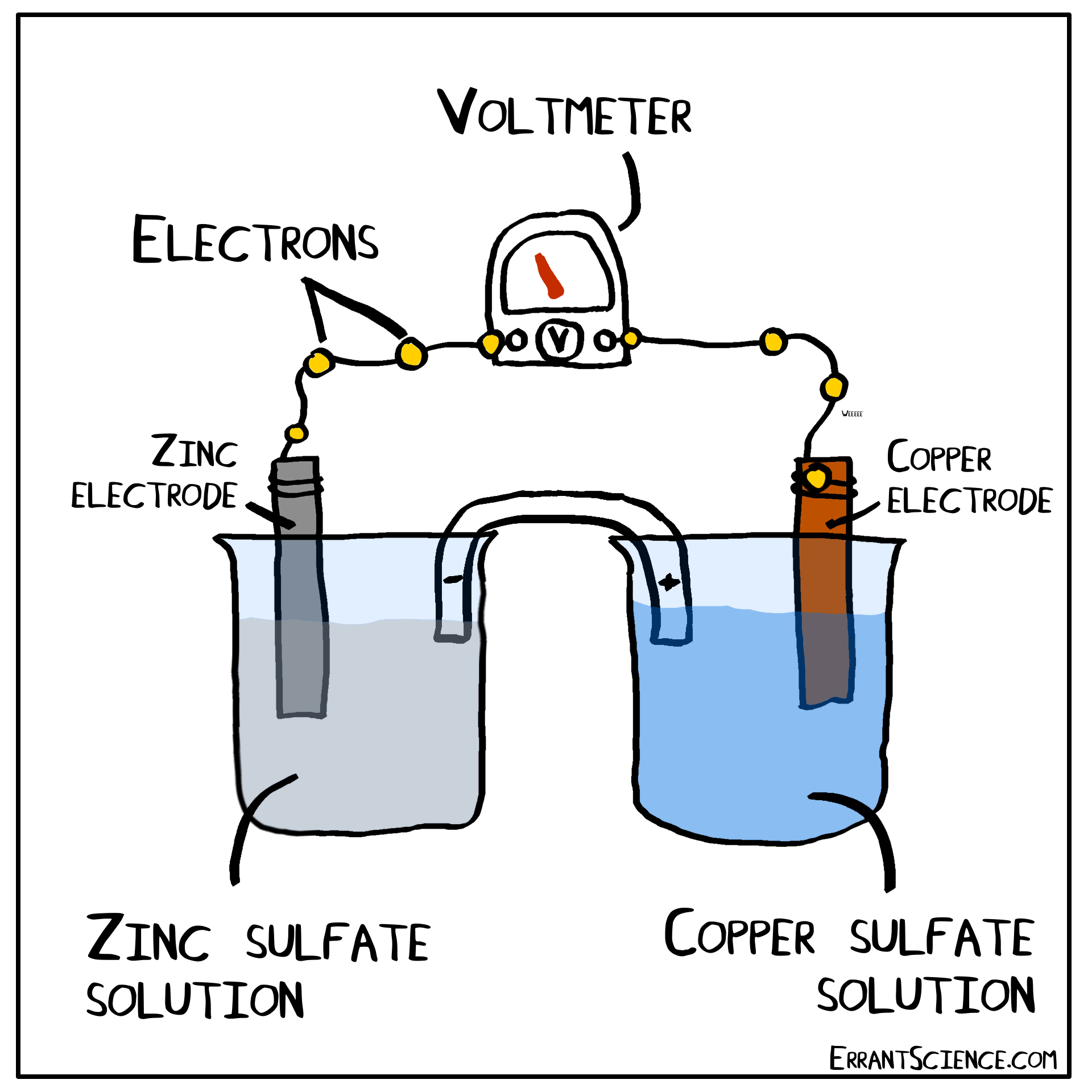
In a similar way, a lemon can be used to create a battery with copper and zinc. Zinc has a more negative electrochemical potential than copper. The zinc therefore loses electrons and copper(II) sulfate is reduced to copper on the surface of the copper. The sulfate (negatively charged) must form a salt with a cation of the salt bridge. If the salt bridge was K2NO3, for instance, than K2SO4 would form. On the other side, Zn2+ forms and this cation can form ZnNO3, with the salt bridge. The anions in the salt bridge keep the side which loses electrons neutral and the cation does the same for the side which gains electrons.
Lemon batteries
A lemon will also serve as a container for electrolyte, allowing the conduction of ions, but not much electricity.
Applications of Batteries
Uses of batteries in pacemakers
Whilst it’s convenient to have so much technology at our fingertips through our smartphones (powered by lithium ion batteries), lithium batteries are a matter of life and death for those with pacemakers. Read this sheet about what came before the batteries.
Why do cells have two half cells and a salt bridge?
The following are more challenging electrochemistry explanations, but well worth a look to understand why a battery is considered to have two half cells and cells need a salt bridge.
And to understand why the salt bridge is so important, learn about Coulombs law (extension topic).
Syllabus links to batteries
We are creating specific resources for batteries. We have started with A level with AQA, which covers lithium batteries and alkaline batteries.
Fuel cells
A fuel cell is a type of electrochemical cell where the reactants for two reactions are provided and the reactions result in an electric current.
In the hydrogen fuel cell, hydrogen is oxidised and oxygen is reduced.
Here, oxygen is reduced with electrons to form hydroxide and at another electrode, the hydroxide ions react with hydrogen to give water and electrons.
In an alkaline cell, there are lots of hydroxide ions around to provide something to oxidise.
By continuously providing the gases needed for these reactions, they can keep going at the electrode, so there is a difference in potential between them. Electrons flow from high potential to low potential through an external circuit, where they do useful work.
The potential is established by the reaction, so the cell needs a constant supply of hydrogen fuel. But it doesn’t need recharging like a battery.
One of the most popular designs is the Alkaline Fuel Cell, so good, they were taken to the moon. Hydrogen fuel cells offer a way of producing electricity using hydrogen, which can be stored in a range of ways. The hydrogen fuel cell only produces water as a product, another useful feature for heading into space.
We hope you have enjoyed your tour through our electrochemistry explanations and videos. If there are any other electrochemistry explanations you would like, please mailto:ner1f18@soton.ac.uk”=””>get in touch.
We have also included electrochemistry explanations for some of what the electrochemistry folks in chemistry at the University of Southampton are up to on our Electrochemistry Research at Southampton page.
