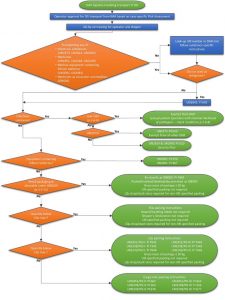
Within a medical context, such payloads could include pharmaceutical products (e.g., medications), patient diagnostic specimens, medical equipment (e.g., defibrillators), or blood or organs for transfusion or transplant. The international regulations governing the air transport of DG are set out in the ‘Technical Instructions for the Safe Transport of Dangerous Goods by Air’ (known as the TI), published biennially by the International Civil Aviation Organization (ICAO). These regulations provide comprehensive requirements for the 3,000+ DG-classified substances, encompassing elements such as packaging, quantity limits, documentation, loading, personnel training, and emergency response protocols.
A comprehensive review was conducted by the E-Drone project into how the current DG regulations can be interpreted and adhered to by drone operators. The review found that the regulations have developed exclusively from the perspective of crewed aircraft, in particular the large, fixed-wing, airliner-type aircraft used to transport nearly all air freight, and the application of DG regulations to drones is an emerging legislative arena, still under development in different regions around the world, leading to uncertainty and the need for authorities to issue DG approvals to drone operators on a case-specific basis, which is a resource-intensive and time-consuming process for everybody involved. In general, there is an urgent need for explicit provisions to be included in future versions of the DG regulations to clarify how they should be applied to drone logistics.
However, supplementary drone-specific regulations have been implemented within the European Union (effective early 2021) and also adopted in the UK. These regulations mandate the carriage of DG packages within Crash Protected Containers (CPCs), which are designed to prevent the release of contents even in the most severe scenarios, such as drone crashes or a dropped container. This increased level of protection was considered necessary because drones, typically, do not undergo the same rigorous certification processes as crewed aircraft (i.e., aircraft and operator certification, pilot licensing) and are therefore seen as more likely to experience an accident.
Members of the E-Drone project team contributed to the consultation with the authorities on developing a procedure for approving CPCs in the UK. This approval procedure involves dropping a container from typical drone operating heights (e.g., 400 ft) onto a hard surface (typically replicating the properties of a public highway). To pass the test, the container must prevent any leakage of contents and sustain minimal structural damage or visible breaches upon impact. The approval procedure is still evolving (in 2024), and the E-Drone project team have been involved in some of the first real-world drop-testing of industry-standard medical containers, with this practical experience being provided as feedback for refinement of the procedure.
Grote M, Cherrett T, Oakey A, Royall P G, Whalley S and Dickinson J (2021) ‘How Do Dangerous Goods Regulations Apply to Uncrewed Aerial Vehicles Transporting Medical Cargos?’, Drones, 5(2), 38.
https://doi.org/10.3390/drones5020038
McLeod F, Cherrett T, Oakey A, Theobald K, Waters T, Grote M, Armstrong J, Denny J and Murray A (2024) ‘Investigating the Crash Protection Performance of a Medical Carrier Bag for Drone Transport’, Logistics, 8(1).
https://doi.org/10.3390/logistics8010031
Oakey A, Grote M, Royall P G and Cherrett T (2022) ‘Enabling Safe and Sustainable Medical Deliveries by Connected Autonomous Freight Vehicles Operating within Dangerous Goods Regulations’, Sustainability, 14(2).