Developments in new gene editing techniques provides stem cells with the ability to bypass the immune system offering new applications in cell replacement therapy.
There are more than 10 million people worldwide currently living with Parkinson’s disease and 3 million people recorded to be living with type 1 diabetes globally in 2017. Both of these chronic diseases are currently incurable and require regular medication and treatment to control. Due to their life-long impacts, many people can relate to the implications these diseases have on both the individuals diagnosed and the family members or friends of the individuals. The negative effects can be physical, mental, social or financial and often a collection of them all.
So if there was a possible solution would you take it?
Research has suggested a new strategy that could provide an endless supply of replacement body parts for individuals suffering from debilitating disorders and diseases. Scientists can now grow stem cells in the laboratory and engineer them into specialised cell types. Which can eventually be transplanted into humans and potentially cure diseases, once believed to be incurable. For Parkinson’s disease this could mean cultivating neurones to combat the progressive damage made by the disease over the years to different parts of the brain, or for type 1 diabetes insulin-producing pancreatic cells could completely reverse the effects of the disease and lastly heart muscle cells to could be transplanted to enhance cardiac function. These are just a few examples of the life-changing effects this new treatment could have.
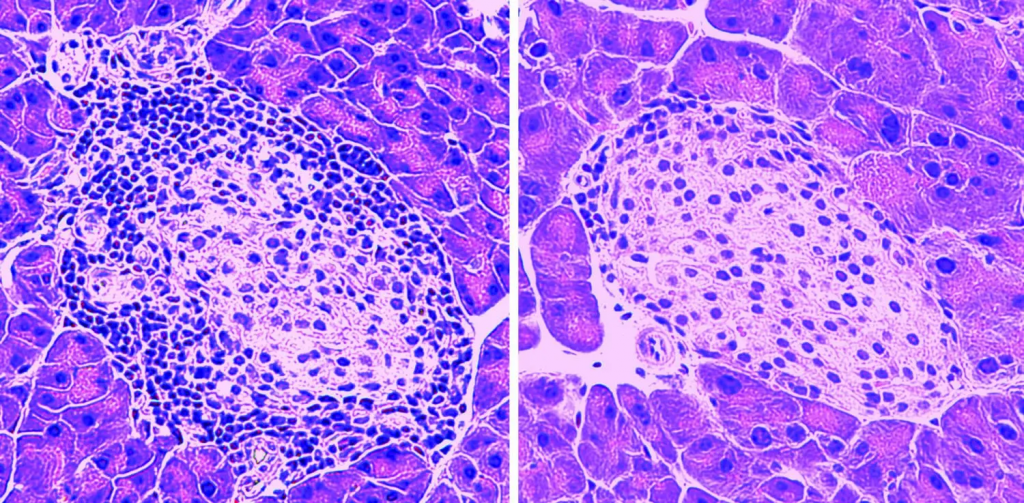
In genetically modified mice predisposed to autoimmune diabetes, pancreatic cells undergo infiltration and destruction by “killer” T-cells, leading to a decline in insulin production (pictured on the left). However, administration of MOTS-c injections mitigated T-cell infiltration, consequently averting disease onset (pictured on the right).
Credit: Newcomb (2021)
How this is possible…
Utilising gene-editing techniques like CRISPR-Cas systems, stem cells can be manipulated to possess immune-evading traits, effectively bypassing recognition mechanisms. Moreover, these engineered cells can integrate fail-safe features to guarantee cells can be eliminated in the case of unforeseen issues. Consequently, such ‘stealth’ cells hold promise to support various cell-replacement therapies.
In most cases, the process starts with the disruption of at least one component of the cell’s major histocompatibility complex (MHC). This complex functions like a molecular identity card, showcasing distinct cellular information fragments that inform the immune system’s T lymphocytes, its frontline defenders, and whether the cell is hostile.
To mitigate potential susceptibility to natural killer cells (NK), certain researchers have suggested the reintroduction of genes encoding particular MHC antigens. These antigens enable the cell to modulate NK cells without eliciting T-cell responses that may induce apoptosis (cell death). NK cells serve as the effector lymphocytes of the innate immune system, tasked with regulating various tumour types and microbial infections to restrict their dissemination and consequent tissue harm. Alternatively, other strategies may involve introducing genes that produce ‘checkpoint’ proteins, specialised molecules aimed at directly suppressing NK cell activity.
Are there any downfalls to this ground-breaking new strategy?
Unfortunately, therapies stemming from stem cells require customisation for each patient, a process that is both time-consuming and costly. Alternatively, these treatments can utilise donor cells; however, due to the tendency of the immune system to reject foreign cells, such ‘allogeneic’ therapies require the administration of immune-suppressing medications alongside treatment. However, this approach escalates the risk of complications like infection and cancer.
Ultimately, the optimal safety strategy, as well as the ideal extent of gene editing required to suppress immune responses, may vary depending on the disease. For instance, pre-made cell therapy for cancer may not require the same design features as one tailored for diabetes, given the differences in the immune system’s response and the distinct risk-benefit considerations for each ailment. In essence, there is no ‘one-size-fits-all’ solution.
With the true test of human trials likely to follow soon the future of this treatment is looking hopeful.
Acknowledgements:
Dolgin, E. (2024). Stealthy Stem Cells to Treat Disease. Nature. [online] doi:https://doi.org/10.1038/d41586-024-00590-y.
Green, A. (2008). Descriptive Epidemiology of Type 1 Diabetes in Youth: Incidence, Mortality, Prevalence, and Secular Trends. Endocrine Research, 33(1-2), pp.1–15. doi:https://doi.org/10.1080/07435800802079924.
Newcomb, B. (2021). Small Protein Protects Pancreatic Cells in Model of Type 1 Diabetes. [online] USC Leonard Davis School of Gerontology. Available at: https://gero.usc.edu/2021/08/12/mots-c-mitochondria-type-1-diabetes/ [Accessed 5 Mar. 2024].
Parkinson’s Foundation (2024). Statistics | Parkinson’s Foundation. [online] www.parkinson.org. Available at: https://www.parkinson.org/understanding-parkinsons/statistics#:~:text=Parkinson.
Vivier, E., Tomasello, E., Baratin, M., Walzer, T. and Ugolini, S. (2008). Functions of Natural Killer Cells. Nature Immunology, 9(5), pp.503–510. doi:https://doi.org/10.1038/ni1582.
Very well written, with an excellent format and images. You’ve included interesting statistics and related it to personal ideas which…
This is a very well written blog, the format is as if you are talking directly to me. The ideas…
Love the Batman GIF :)
This is an excellent, well written blog. The narrative is engaging and easy to follow. It could be improved by…
This is a well-communicated blog. The it is written well with good use of multimedia. It could be improved with…