What would you do if you were offered the chance to enhance your future child’s intellect and athletic abilities before they were even born?
Would you do it?
With the rapid advancements in genetic engineering, this question may demand an answer sooner than we first thought!
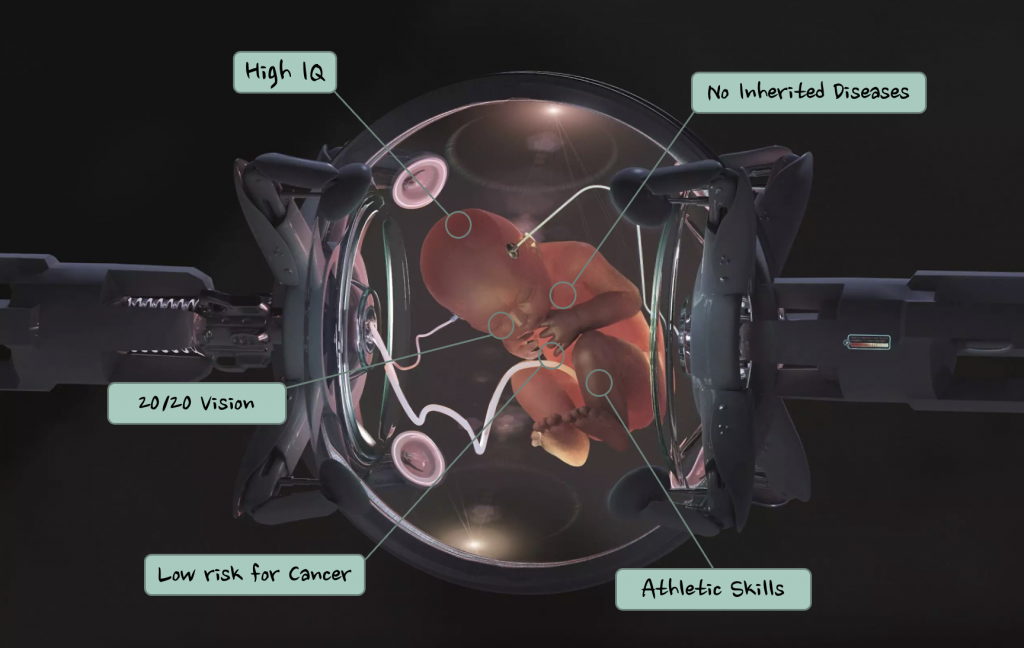
What is Genetic Engineering?
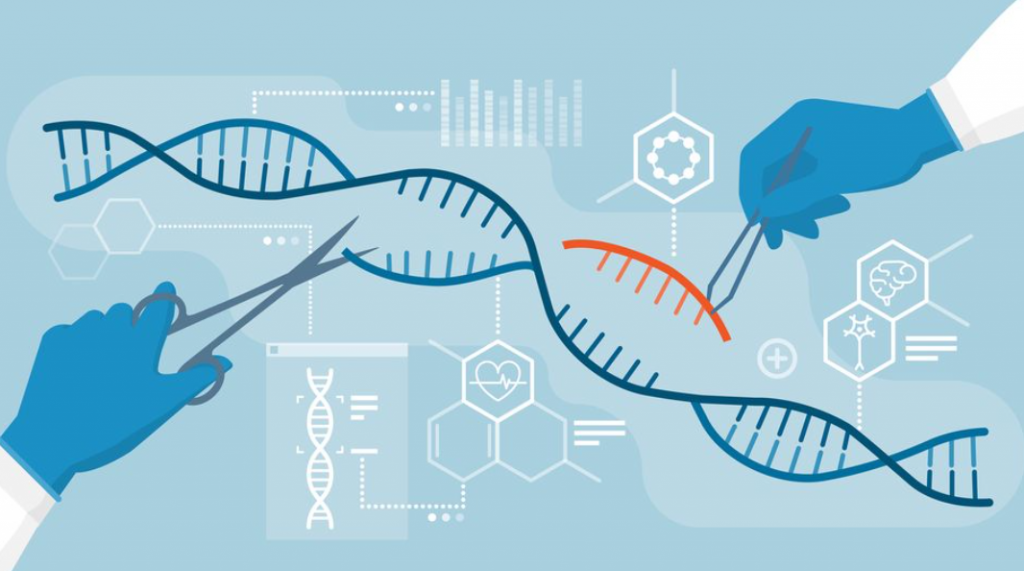
Genetic engineering is the process of deliberately manipulating and modifying the genetic makeup of an organism (the genetic makeup being the stuff that makes us… well… us). At the forefront of this endeavour is CRISPR-Cas, a ground-breaking technology developed in 2012. CRISPR-Cas allows scientists to target specific DNA sequences with remarkable precision and altering their function.
The potential of genetic engineering is immense, extending far beyond what you might imagine. It has been pivotal in treating human diseases such as diabetes, sickle cell disease and haemophilia.
This all sounds great, doesn’t it? Individuals grappling with diseases may not have to struggle anymore!
However, while the prospects of genetic engineering may seem promising, there looms a shadow of ethical uncertainty. The fact we can modify human cells to change their function means we can also target and modify human embryo cells.
We can basically design a human.
You may think this idea is far-fetched, and I wouldn’t blame you! The concept of designing human traits to align with our preferences may sound like the plot of a science fiction film, but it’s a reality within reach. This power to tailor human traits come with ethical risks and concerns that cannot be ignored.
Societal Implications
The societal implications of genetic engineering are vast and complex. The ability to shape human traits could worsen existing social inequalities as access to genetic enhancements may only be available to the wealthy. Additionally, there are significant concerns about eugenics, which involves altering genes to improve human traits. These actions could potentially redefine the very essence of humanity. It begs the question: Is it ethically okay to attempt to ‘play god’?
“The cloning of humans is on most of the lists of things to worry about from Science, along with behaviour control, genetic engineering, transplanted heads, computer poetry and the unrestrained growth of plastic flowers.”
– Lewis Thomas
Conclusion
Given the exciting potential of genetic engineering, we need to be careful. While it could help reduce human suffering and improve medicine, it also brings up ethical questions and societal issues that we need to think about. As the possibilities of genetic engineering are being explored, we have to make sure we’re guided by ethics and a concern for everyone’s well-being. We can use genetic engineering to make life better for everyone, not change what life is like completely.






Very well written, with an excellent format and images. You’ve included interesting statistics and related it to personal ideas which…
This is a very well written blog, the format is as if you are talking directly to me. The ideas…
Love the Batman GIF :)
This is an excellent, well written blog. The narrative is engaging and easy to follow. It could be improved by…
This is a well-communicated blog. The it is written well with good use of multimedia. It could be improved with…