Green fluorescent protein (GFP) is a fluorescent protein discovered in Aequorea Victoria that I’ve found to have many fascinating applications in biological research and clinical scenarios due to its central chromophore within its beta-barrel structure. I conducted a review paper in the ‘exploring proteins’ module focusing on the structure and functions of GFP and emphasised its benefits such as in tumour recognition however, I had a perspective shift upon discovering an article click here that explored previously unknown harmful outcomes of certain GFP strains have on transgenic organisms.
GFP in hematopoietic stem cell tracing
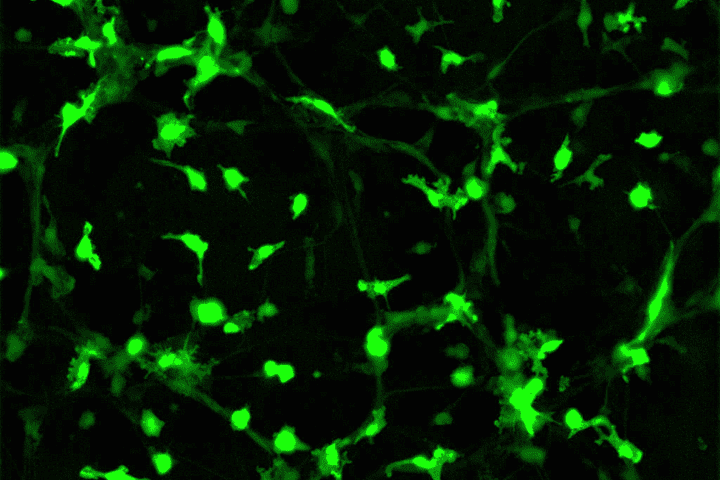
I have a passion for neuroscience and its intricacy including glial helper cells and their functions. Microglia are intriguing as they’re the immune cells of the brain protecting from pathogens and neurodegeneration. Hematopoietic multipotent stem cells produced in the bone marrow are released into the brain and differentiate into microglia cells in response to damage. Through transgenesis, the process by which foreign genetic material is introduced into an organism, GFP has been transduced into hematopoietic stem cells enabling us to track the movement and differentiation patterns as they move to the site of injury.
The use of mutated GFP (enhanced GFPs) combined with an MSCV vector (for stability) in mice chimeras illustrated that upon damage, these stem cells with GFP move past the blood-brain barrier and increase in abundance in the brain. Passage through the blood-brain barrier is rare so this may allow us to create gene therapies against neurological disorders and allow drug delivery similar to that of nanodroplets which I was shown when visiting Southampton General Hospital in which drugs can piggyback into these droplets to the site of injury therefore, something similar could be used using microglia. I think this demonstrates how powerful GFP can be in terms of disease treatments with around 6 million individuals in the US alone being impacted by these types of disorders as shown in this review article the need for this type of research is evident.
The disadvantages of GFP strains
Several new research sources have informed me of the harm GFP expression can cause to transgenic organisms. Several GFP variants show cytotoxic, immunogenetic, and physiological changes to the cardiovascular, urinary tract, and CNS some of which cause death such as in the mice strain alpha-myHC-EGFP which develops dilated cardiomyopathy where the left ventricle of the heart is weakened. The deterioration of GFP as it fluoresces also prevents accurate prolonged tracking. This means the research gathered using GFP in development such as in microglia may not be interpreted correctly. The ASPA Act of 1986 lays out fundamental requirements fulfilled to justify transgenic research projects. GFP is complicated since we still don’t understand which GFP variants cause damage to vertebrates so the rules are less defined.
My views on GFP usage
I think GFP is an incredible protein with the potential to have large impacts on a range of disease treatments and understanding of how the components of the body function and develop however, I also feel the need to emphasise the requirement for research into the different GFP strains and the changes they cause to transgenic animals so that we can properly utilise GFP without the added side effects. GFP in stem cell research has obviously been remarkable allowing us to show the mechanism by which the immune cells develop and create opportunities for treatments that would have a huge impact on a large percentage of the population yet the unsettling amount of unknown knowledge of GFP limits what research I think should be carried out by GFP until further information is obtained.



Very well written, with an excellent format and images. You’ve included interesting statistics and related it to personal ideas which…
This is a very well written blog, the format is as if you are talking directly to me. The ideas…
Love the Batman GIF :)
This is an excellent, well written blog. The narrative is engaging and easy to follow. It could be improved by…
This is a well-communicated blog. The it is written well with good use of multimedia. It could be improved with…