Stem cells have always been an intriguing topic to me, particularly their therapeutic potential. The idea of being able to use one undifferentiated cell to create whole new organs or body parts is fascinating. After our lecture on stem cells and their therapeutic use, I decided to research the different ways that stem cells are currently being used in medicine, and one case in particular stood out.
The Story
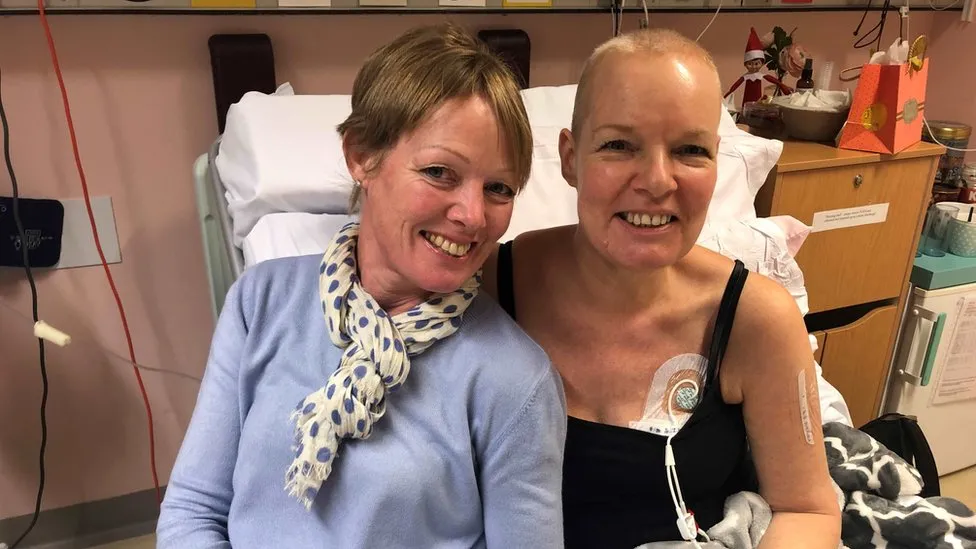
Lesley Calder was diagnosed with acute myeloid leukaemia in 2019. Chemotherapy treatment was unsuccessful for her cancer, and she was left with the only option of a stem cell transplant. Lesley’s three siblings volunteered to be tested, with the slim chance of finding a sibling match (~25%). By some miracle, 2 siblings were full matches and 1 was a half match. Lesley’s sister Annie was chosen to be the donor, and amazingly, Lesley has since made a full recovery.
Using Stem Cells to Treat Cancer
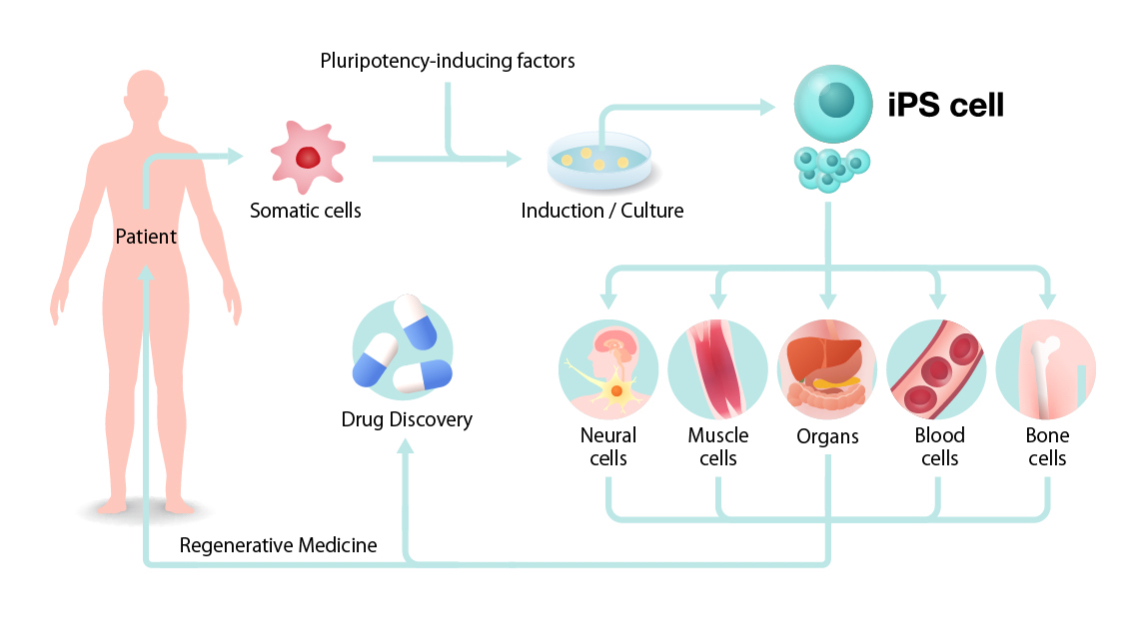
A stem cell is defined as a cell that can self-renew indefinitely and has the capacity to differentiate into many cell types. Their normal function within the body is to replace old, damaged or defective cells to maintain normal tissue function.
Stem cell transplants are used to treat diseases where the bone marrow is damaged or defective, meaning that healthy blood cells can no longer be produced. This is the case in blood cancers (e.g. leukaemia and lymphoma), which primarily affect white blood cells. The loss of blood cells is further exacerbated by intensive cancer treatment (e.g. chemotherapy), which can also damage/destroy healthy cells. The transplantation of stem cells produces new blood cells, and helps to defend against the cancer.
Stem Cell vs. Bone Marrow Transplants
Before this research, I had only heard about bone marrow transplants, and didn’t know stem cell transplants existed, which made me wonder what the difference is. They are essentially the same thing, but differ in the locations where the cells are collected. A stem cell transplant involves collecting stem cells from the bloodstream, which is less invasive than a bone marrow transplant, which involves collecting a person’s bone marrow from within their bone (usually pelvic).
Information from Cancer Research UK says that stem cell transplants are the more common of the two, which I found surprising, considering I hadn’t heard of them. This is because stem cell transplants are: less invasive, easier to perform, have a higher yield of cells and have a quicker blood count recovery.
The Problem & Final Thoughts
Through this research, I have found that over 70% of patients who require a stem cell transplant will not find a compatible donor in their family. Additionally, only 3% of the UK population (and <6% of people in Northern Ireland) are registered to be stem cell donors, making the chances of finding a compatible match even lower. For the majority of patients, like Lesley, a stem cell transplant is their only chance at recovery, and their chances of success are slim.
Lesley’s story prompted her son Max to join the stem cell donor register in hopes of helping others like his mum, and he has already been called upon to donate. By sharing her story, I hope to inspire others to join the register as well, as I will definitely be doing. Anyone aged 17-55 and in good health can sign up here. For more information on stem cell transplants, visit NHS, Cancer Research UK or Leukaemia & Lymphoma Society.

:max_bytes(150000):strip_icc()/senior-woman-with-heart-attack-and-headache-fall-down-at-home-1183594716-b5da91d0fe9b45638d546820fafe8d13.jpg)













Very well written, with an excellent format and images. You’ve included interesting statistics and related it to personal ideas which…
This is a very well written blog, the format is as if you are talking directly to me. The ideas…
Love the Batman GIF :)
This is an excellent, well written blog. The narrative is engaging and easy to follow. It could be improved by…
This is a well-communicated blog. The it is written well with good use of multimedia. It could be improved with…