The concept for my summative blog emerged during my dissertation lab project, where I used HepG2 cells to assess the hepatoprotective impacts of chlorogenic acid against paracetamol induced liver toxicity. During this process I became familiar with cell culture, treating and assaying them most days, but when I came to seed the cells myself and prepare new culture medium, my attention was brought to foetal bovine serum. When researching the topic I found interest in the ethical debate surrounding the production of foetal bovine serum and the demand for alternatives.

Foetal bovine serum (FBS) is a key component of cell culture medium and is used extensively for research applications. It contains essential hormones and growth factors providing a rich culture system; supporting the widest range of cell types, including cell lines and primary cells.
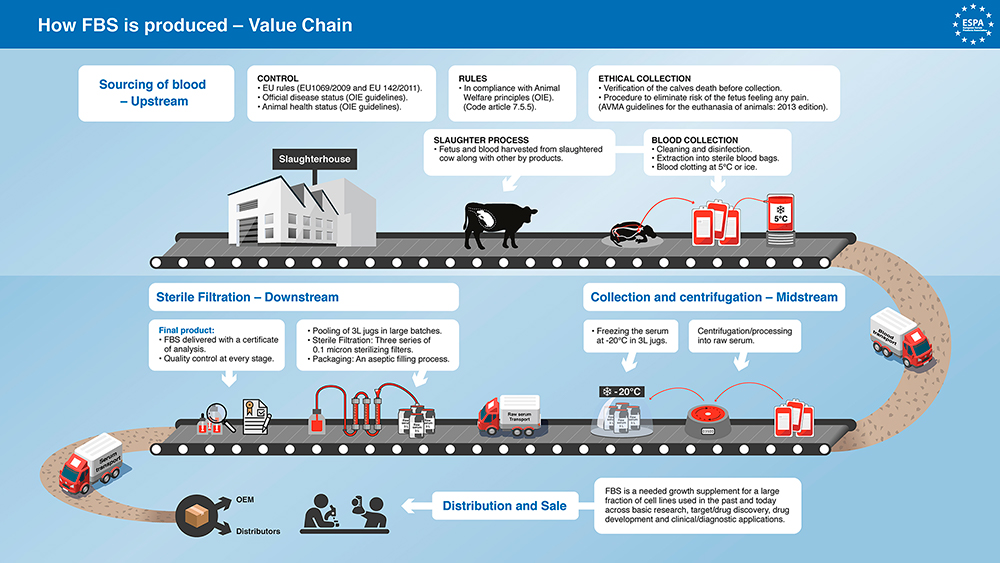
Although figure 1 provides a thorough overview of the FBS production process, it fails to describe the actual harvest, the point of most concern. FBS is typically obtained by the cardiac puncture of cow foetuses sourced from cows found to be pregnant after slaughter. This is carried out once the foetuses are declared deceased and without the administration of anaesthesia.
An article from 2002 covers the proposed reasons behind the ethical concerns surrounding FBS production and the potential for foetuses to experience pain during the process. One of the primary ethical concerns regarding the harvesting procedure involves the resistance of mammal foetuses to anoxia; the complete loss of oxygen supply to the body or brain. Mammals possess protective mechanisms allowing maintained brain function even after oxygen deprivation. These mechanisms include the ability to meet energy requirements via anaerobic respiration, the prioritisation of blood flow to critical organs such as the brain, and a reduced oxygen demand in the foetal brain, compared to adults. It is expected that the bovine foetuses have normal brain function at time of cardiac puncture, allowing us to assume that they are aware of the procedure and experience pain as a result. The paper then goes on to explore the timeline by which the cow foetus develops nociception and the ability to experience pain.
Since this paper is now over 20 years old I looked for more recent advancements. This led me to an article addressing the animal welfare ethics concerning FBS production and how the industry has altered it’s practises to avoid such concerns.
To summarise, the article discusses the debates regarding foetal sentience and consciousness, raising concerns about potential suffering during the procedure and proposes steps to minimise harm. They specifically make reference to the European Food Standards Agency (EFSA) recommendation to immediately open the uterus, stun and kill the foetus upon detecting pregnancy in a slaughtered cow. This ensures the foetus is unconscious and therefore doesn’t experience pain during the blood extraction. They suggest increased monitoring within the serum industry to ensure compliance with ethical standards and that by implementing such measures, the industry can demonstrate its commitment to ethical practise. Like the 2002 paper, they also conclude that it is time for researchers to adopt more modern approaches to human cell culture-based research and to move away from the use of FBS.
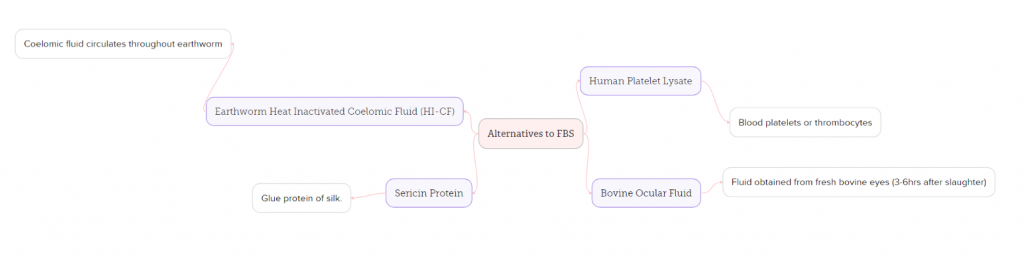
To conclude, at present time the production of FBS is deemed to be up to ethical standards providing individual producers follow legislation and guidelines provided. It’s clear that to fully address the concerns around animal welfare, alternatives to FBS are required. By adopting such alternatives, we can achieve greater scientific accuracy whilst demonstrating stronger ethical consideration.
References:
Jochems, C.E.A., van der Valk, J.B.F., Stafleu, F.R. and Baumans, V. (2002). The use of fetal bovine serum: ethical or scientific problem? Alternatives to laboratory animals : ATLA, [online] 30(2), pp.219–27. doi:https://doi.org/10.1177/026119290203000208.
McCann, T.J. and Treasure, C. (2022b). Addressing Animal Welfare Issues in Fetal Blood Collection for Fetal Bovine Serum Production. Alternatives to Laboratory Animals, 50(5), pp.365–368. doi:https://doi.org/10.1177/02611929221117992.

Very well written, with an excellent format and images. You’ve included interesting statistics and related it to personal ideas which…
This is a very well written blog, the format is as if you are talking directly to me. The ideas…
Love the Batman GIF :)
This is an excellent, well written blog. The narrative is engaging and easy to follow. It could be improved by…
This is a well-communicated blog. The it is written well with good use of multimedia. It could be improved with…