As a registered organ donor before the opt-out system, I could not fathom why people would not want to donate their organs. It baffled me, and yet in 2019, I was in a debating competition where I had to oppose the idea of organ donation as an opt-out system – my point was simple. Surely the argument that this is a violation of a donor’s autonomy and implies given consent, would always triumph over the utilitarian view, that it should be the greatest good for the greatest number, right?

In 2020, the UK introduced a new law, the ‘opt-out’ system. Many believed this diminished a donor’s autonomy, yet, by definition, there is still a choice in opting out. The new law made sense to me, when around 6,945 people are currently awaiting a transplant in England, I found it hard to understand the counter-argument.
Dr. Jon Dawson covered the ideas of organ donation and autonomy throughout his lectures. I saw a wide range of views in one group of students of a similar age and with the same privilege of higher education. Dr. Dawson put forward the Alder Hey case, where about 850 organs were being harvested after death without any form of consent from either the patients or guardians.
There was a large consensus that this was not okay from the class, the lack of adequate consent when removing organs and tissue from patients was barbaric, nonetheless arguments can be made that people uneducated in the opt-out system are therefore giving ill-formed consent.
This case made me think of the book Never Let Me Go by Kazuo Ishiguro, where clones are created for the purpose of organ donation, and once they have donated around three of their organs, their short lives are over. Although this dystopian novel seems far stretched, the premise behind it still stands. Especially as since 2015, advancements in tissue engineering has shown animals as a viable surrogate for growing organs.
In 2019, Hiromitsu Nakauchi had the first approved experiments to allow a human-animal hybrid to grow fully. This sounds like some werewolf science-fiction, Morbius esc (awful movie); however, this could be the key to the current organ shortage. In an ideal world, we could grow the organ required at the drop of a hat- but here is a scientific solution where we could grow organs within animals and harvest them without invasive surgery on humans, or an ethical debate of autonomy.
Now, PETA and animal rebellion may be opposed to this idea, but I think animals will forever hold a place in scientific research, so could this be a legal viable solution? What do you think?
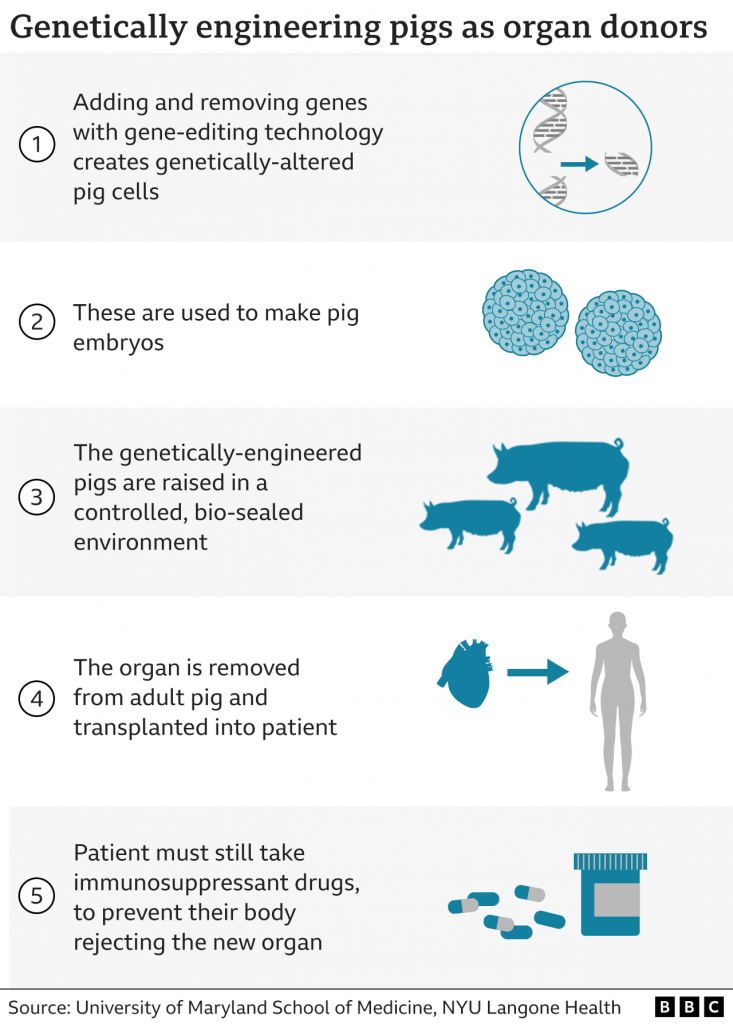
Well, in January 2022, the first pig to human transplant was done; a genetically engineered pig’s heart was harvested and placed into a patient. Although this required a lot of medication and extra resources, the heart did work prolonging the patients life for two months. This was a major breakthrough- can we now combat our organ shortage through animals?
Whether genetically modified pigs or human-animal hybrids hold the future in organ donation, ethics must be considered- we can not return to being so desperate as to take organs without consent. The podcast below is an informal discussion on organ donations with the opinions of two biochemists discussing frankly the possible future of organs, ethics and consent for further insight into this medical and ethical minefield.
This is an initially reflective and well researched blog showing how you have chosen to explore the emerging field of…
This is a good attempt at a blog, where you reflect on your recent learning at a lecture/workshop to describe…
This is a fair to good blog, reflecting on your recent learning in some of your modules. You provide a…
This is an engagingly written and reflective blog focussed in general on ethics in medicine. You might improve by citing…
This is a good and well written an presented blog on an original subject - biofilms on implants. You explain…