World First:
The first human/animal chimera was a human/rabbit chimera documented in Cell Research 2003 where the scientists from Shanghai Second Medical University fused human skin cells with rabbit eggs and allowed them to develop in laboratory dishes for several days before their human embryonic stem cells were harvested. This raises many ethical issues, specifically with embryonic stem cell harvesting as many people see the destruction of the embryo to retrieve these cells to be ending a human life, and some scientists even argue the research is not necessary in the first place.
Primate Chimeras:
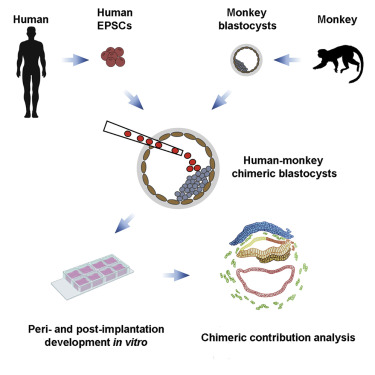
The negative reaction to the 2003 paper did not deter a team of researchers from China, Spain and the USA from creating the first human/monkey chimera in 2021, who injected human epithelial pluripotent stem cells (hEPSCs) into macaque blastocysts.
This video shows the growth of one of the chimeric embryos, with the human cells highlighted in orange where you can see them migrating and undergoing mitosis.
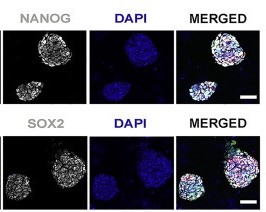
In over half of the injected embryos TD+ human cells were found within the embryonic disc which is responsible for detaching embryonic cells from the blastocyst walls and forms a trilaminar embryo- an important step in embryonic development.
Ethical Considerations:
As it stands at the embryonic stage of development there are already ethical concerns with regard to the harvesting of embryonic stem cells from these chimera embryos, as some consider this to be killing a living organism, however if these cells were allowed to grow and able to produce an adult organism the concerns become even more sinister- organ farming.
Growing human organs in animals for the sole purpose of transplanting them into awaiting human patients is a conflicted topic for many reasons. Jehovah’s Witnesses famously refuse blood transfusions, and many more would likely object to receiving an organ grown inside an animal. The possibility of growing human organs using the patient’s own cells may persuade more, but many would still object to receiving an organ grown inside of an animal. Furthermore, there are research limitations on primates due to their similarity with humans, but it is this very similarity which could make them one of the best candidates for organ farming.
On the other side of the fence, you could argue that harvesting organs from animals like monkeys and pigs is no different than farming any other sort of animal product, with the added benefit of saving lives. One of the considerations with chimera organ harvesting is which animals we create chimeras from. Monkeys are typically thought in the west to be too intelligent to eat, and many religions disallow the consumption of pork so would likely refuse organs from one too, however pigs and monkeys are typically viewed as the best vessels for growing human organs. If the animal used is already farmed en masse, how bad is it really?
According to the HRSA website, there are 104,234 people in the US on the national transplant waiting list as of 24/03/23, and 17 people die every day waiting for an organ transplant. over 42,000 organ transplants were performed in the US in 2022. If human/monkey chimera technology advanced to the point of transplanting mature organs into human recipients, it could help to alleviate the organ crisis we face in the world today.
Human/animal chimeras for organ harvesting could save thousands in the future, but is it worth sacrificing animals to play God?
This is an initially reflective and well researched blog showing how you have chosen to explore the emerging field of…
This is a good attempt at a blog, where you reflect on your recent learning at a lecture/workshop to describe…
This is a fair to good blog, reflecting on your recent learning in some of your modules. You provide a…
This is an engagingly written and reflective blog focussed in general on ethics in medicine. You might improve by citing…
This is a good and well written an presented blog on an original subject - biofilms on implants. You explain…