A lecture by Dr. Alex Dickinson on prosthetic implants highlighted how patient satisfaction rates are a rationale for continued research into implants. I remember learning in Year 2 about how biofilms were one of the major difficulties faced when it came to implants and wanted to look more into what is being done to combat this.
According to the National Joint Registry in 2013, 34% of knee and hip implants had to be replaced due to infection. Infections of implants result in the patient experiencing similar symptoms to before they had the implant, such as swelling in the area, pain and stiffness. I can see how frustrating this is for the patients as it must feel like you have gone back to square one. Furthermore, replacing an implant is quite costly and takes up time that could have been used for new patients who require the implant in the first place, not to mention the invasiveness of the treatment.
How does the infection occur ?
In able to understand how treatments are manufactured, I needed to understand how the infection occur in the first place. From my own knowledge, I know that bacteria and other microorganisms enjoy smooth surfaces where they attached on to and form biofilms. Here they form communities where they ‘live’ which has a constant and stable supply of nutrients to support their survival.
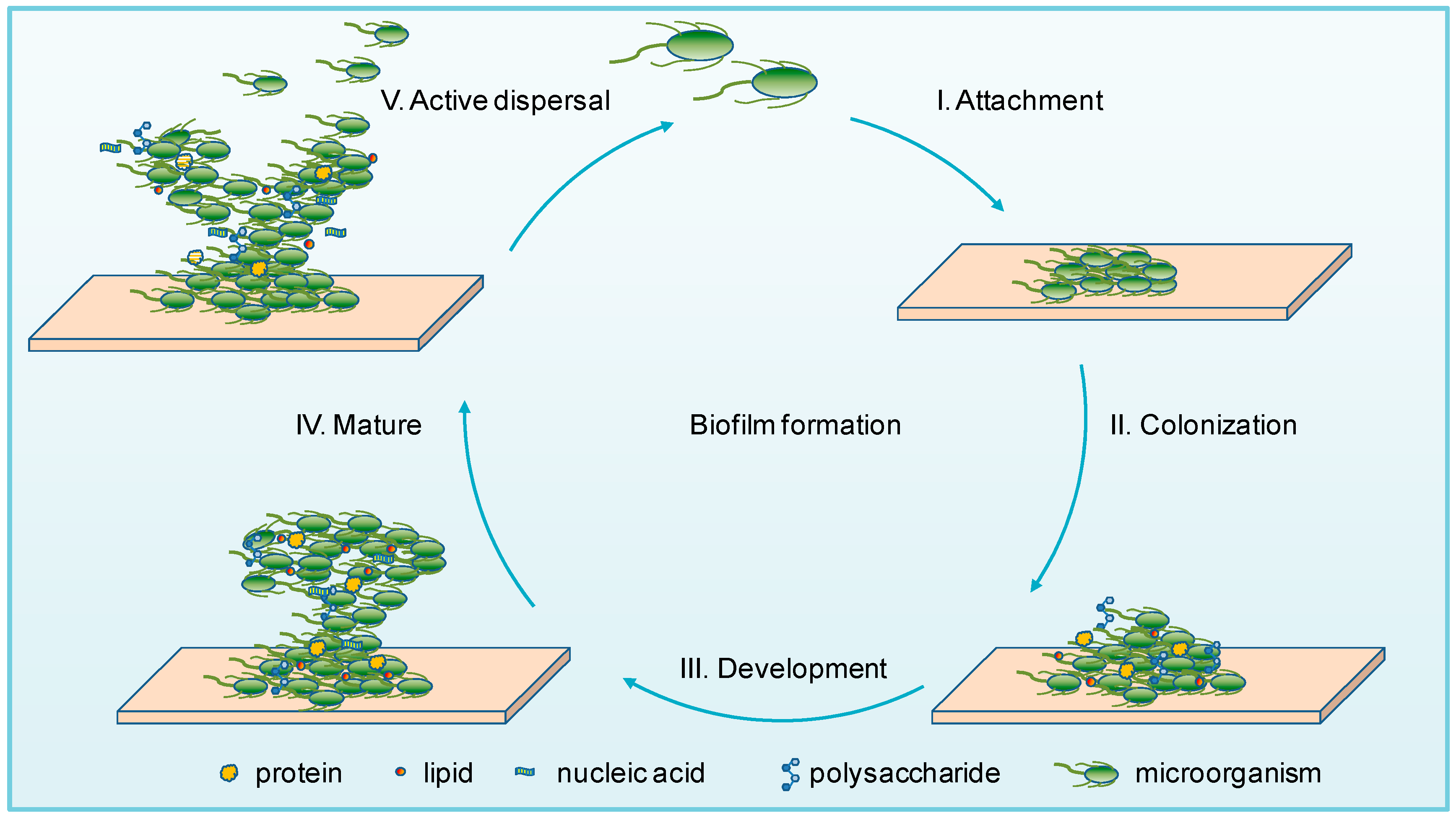
What I did not know is that there are two ways that this infection can occur on the implant. The first being during the surgical procedure – where the bacteria can come from the individuals flora or the operating environment. The second is the microorganisms can be carried by the blood and form a biofilm- this one occurs after the surgery has occurred. In both ways the biofilm maturation occurs over time and it takes some years before it becomes harmful.
By the time the infection is discovered the biofilm has developed so much it becomes difficult for the clinicians to identify what microorganisms that are included in this making it harder to treat them as it is time consuming and requires heavy reach.
What about antibiotics and our immune system ?
Well… multiple studies have shown that biofilms show hight antibiotic resistance due to their formation. When in the biofilm, the bacteria switches off certain genes which could be targeted by these antibiotic rendering them ineffective. Remarkably, one study showed that some bacteria in the biofilm were able express certain phenotypes which would result in the removal of the antibiotics! Due to the lack of blood supply within the implant the immune system is limited.
Current Treatments
So far the most common treatments is DAIR- debridement, antibiotics and implant retention. This procedure involves reopening the implant, washing it out with fluid, removing damage tissue followed by a course of antibiotics , which can vary in duration. The success rates of this treatment vary due to the heterogeneity of the patients, length of infection and type of infection. Typically study’s show that DAIR has a higher success rate with acute infection and when the infection has progressed there is an increased chance of the patients reinfection and them requiring are placement implant. Although there are some success with this treatment , it is still invasive and costly and relies on early detection – which we have seen before is quite difficult.
Future Prospects
I decided to look into research about future therapies or ways to improve the DAIR. I learnt that in order for clinicians to improve the success of DAIR, they are looking into trying to detect the high levels of antibody within the patients so they could be able to intervene at an earlier point. But the invasiveness and time consuming aspects still remain
Antimicrobial peptides have been showing promising results in therapeutics. They already exist in our innate immune system. Their cationic charge allows them kill a range of different microorganisms but not attack the mammalian cells. In addition to this there are other antibiotics such as fluoroquinolones and rifampin penetrate biofilms. This type of treatment ideal but so far researchers have been unable to find a mechanisms for these in vivo.
So if the treatments are lacking, what can be done to prevent this from occurring in the first place? This is where I came across an fascinating paper about looking potential biomaterials which prevent biofilms from forming. The paper looking at what can be done to the biomaterials of the implants to interfere with the biofilm formation. They conducted a lot of experiments on the metal roughness and tried coating different materials onto the implant. Most notably, the rougher the metal and coating the implants with ions made it harder for the biofilm to form. With this information I began to think that perhaps copper , an ion producing mental could be used to make the implants. Well after reading up on this making copper implants would be impractical but researchers are looking into coating the implants with copper – due to its antimicrobial properties
I was particularly interested in coating the implants with an acylase activity which is turn disrupts quorum -sensing. From studying my course quorum sensing is the way bacterial communicates with each other – so being able to stop that would be detrimental in winning the war against biofilms.

Concluding thoughts
Coming into this, I honestly thought that there was not much hope for resolving biofilm infection of implants. This is mainly because there is not really a reliable , non-invasive treatment for this. However in this case I believe the best way beat biofilm is prevention. The biomaterials look highly promising- although a lot of research has to be done to assure that it doesn’t affect human cells. this is definitely an area that I will keep up to date on as there could be huge developments soon.
This is a good and well written an presented blog on an original subject – biofilms on implants. You explain the science and the medical problem well, reflecting on your learning journey throughout.
You could improve by taking a bit more care with your sources (for example, the NJR source is actually a paper, and claims a rate of ~1% infection rather than 24$), and by using some of your own critical analysis to weight up the evidence.