
My first well-formed academic interest took shape in the form of bright-eyed awe at the beauty, diversity, and complexity of every life-form that I set sights on. How wonderful the pattern of that tree’s branches! I would wonder at the factors driving the formation of that pattern.
This might all seem to be getting away from the topic of tissue-engineering, but morphogenesis (how the shape of organisms arises during development) is of great relevance in research and the clinic! There are two main views of how development occurs, with the classical ‘mosaic’ view, in which cells obey a deterministic programme, their fates determined genetically, and the ‘regulative’ view, in which cell-cell interactions and multidirectional information transfer affect the developmental trajectories of the cell, with evidence pointing that both have their place in different developmental stages, as the cells take in physical, electrical, and chemical cues to “decide” how to arrange themselves. The way that organs and tissues form is at the core of many regenerative medicine issues, from birth defects, to genetic diseases, to cancer. Contrary to the intuitive assumption that organisms with higher regenerative capacity would also have higher propensity for cancer due to their higher cell proliferation, they actually have lower cancer incidences, implying the competent morphogenetic pathways used for regeneration may also prevent cells from falling into the disorder which can lead to tumorigenesis.
Indeed, this ability of cells to self-organise into tissues and organs has been exploited in the form of organoids, mini 3D structures which can be derived from Embryonic Stem Cells (ESCs) and induced Pluripotent Stem Cells (iPSCs), which can have very similar functions to in vivo organs, having incredible potential for drug testing, disease models, and even the possibility of becoming an alternative for organ transplants, overcoming barriers such as long waiting times and tissue rejection in patients; an organ grown from the patient’s own iPSCs, maybe edited genetically into a healthy form if applicable. This might even be an alternative to xenotransplants, bypassing several of the ethical issues associated with using animals for organ harvesting. Of course, organoids come with their own suite of ethical concerns, from source of the stem cells to the moral and legal status of organoids, especially in the cases of multi-organ and brain organoids, but the benefits of organoid research are worth the extra necessary steps to ensure such research follows our moral values.
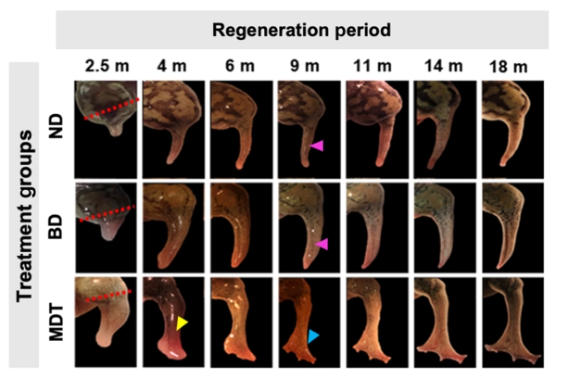
And if that sounds fantastical, imagine my surprise when I found this property of cells could lead to the possibility of replacement limbs, not in the form of prostheses or even grown on scaffolds, but grown by the patient themselves! Over the course of a 2022 study, Murugan et al were able to trigger the regrowth of frog legs with the use of a bioreactor which served to protect the site of injury and deliver a drug-cocktail which, among other things, prevented growth of scar tissue by inhibiting collagen and encouraged nerve, muscle growth, and vascularisation, activating the body’s own regenerative abilities and molecular pathways used during embryonic development. Over the next 18 months, the frogs subjected to the 24-hour treatment grew back almost fully functional legs which they could stand and swim with. Of course, this technology is a long way away from clinical application, with mice being currently used to test whether this approach would even work in mammals. This, and other techniques discussed here, are yet in their infancy, with much of the basic groundwork yet to be done. Still, the future landscape of regenerative medicine holds many incredible possibilities that I am excited to witness.