A lecture on tissue engineering introduced me to the concept of Bioprinting. Although I am fully aware of the idea of 3D printing. I wanted to look more into how biological materials are incorporated into 3D printing to create tools that can be utilised within our bodies. Bioprinting is ‘the use of 3D technology with materials that incorporate living cells’. They are most commonly known for organ growth to battle the organ transplant crisis. But did you know that they are also being used to improve therapeutics, specifically drug delivery? I was particularly drawn to this as it shows potential in making medicine more personalised – a topic that I have always had an interest in.

Bioprinting and its biocompatibility
There are different types of 3D Bioprinting techniques such as stereolithography and fused deposition modelling. However, bioprinting with bioinks is able to produce structures that obtain many properties that can be used to develop structures that can be efficient for drug delivery.
Bioinks are hydrogels that can be made up from substances such as gelatin, alginate and hyaluronic acid – materials that are highly compatible within the body. The composition of the hydrogels can vary and as a result they can be modified so the cross linkage of the polymers can be made reversible or permanent depending on how the materials are treated physically or chemically. These are referred to as methacrylate hydrogels and the cross linkage can be used as a tool to be used to control the release of the drugs within the body. The bioinks can also incorporate synthetic polymers which can be used to further modify their properties such a degradation, increasing the biocompatibility.
Potential therapeutic uses
- Drugs
The main issue in drug therapeutics is the distribution of the drug itself within the body. In addition to this due to individual differences, a set dosage doesn’t work for everyone and a patient may not necessarily take their medication correctly. (Martinez et al., 2017) was able to print drug-loaded hydrogel with water which was able to release the drug at rates that were dependent on the water content. A delivery system, that would combat the improper use of medication, release the drug at the correct site of absorption, and could be further modified to be dose specific for each patient.
With further reading, I discovered that tuberculosis required two drugs for it to be treated, rifampicin and isoniazid. Multiple studies have shown that these two drugs can negatively impact the effectiveness of the other and they are also required to be absorbed in different areas within the body for effective treatment. (Genina et al., 2017) designed an oral dual-compartmental dosage (dcDU) unit via 3D printing and hot melt extrusion

The dcDU was tested in vitro and in vivo using rats, where drug release was analysed and it was noted that sealing and the compartments improved the drug release of each drug. This study particularly opened my eyes to the amazing things that could be achieved with bioprinting, it was here when I begun to realise how much of a big impact on therapeutics that bioprinting would be. An infection that could require a 2-week course of 2 different antibiotics would be reduced to one oral dosage.
- Hormones
Like any other scientist, I began to work what else could we dispense and came across (Tappa et al., 2017). Intrauterine devices are the most common form of long term birth control. Although they are very efficient they are not necessarily personalised to the individual. Polycaprolactone pellets (PCL) were first coated with hormones and infused with silicone oil. Here is prime example of how synthetic polymers are used as the PCL allows for degradation and has a low melting temperature that will not interfere with the high degradation temperatures of the hormone. They then used this to print different 3D hormone-releasing IUDs which suggested promising uses in gynaecology applications.
- Growth factors
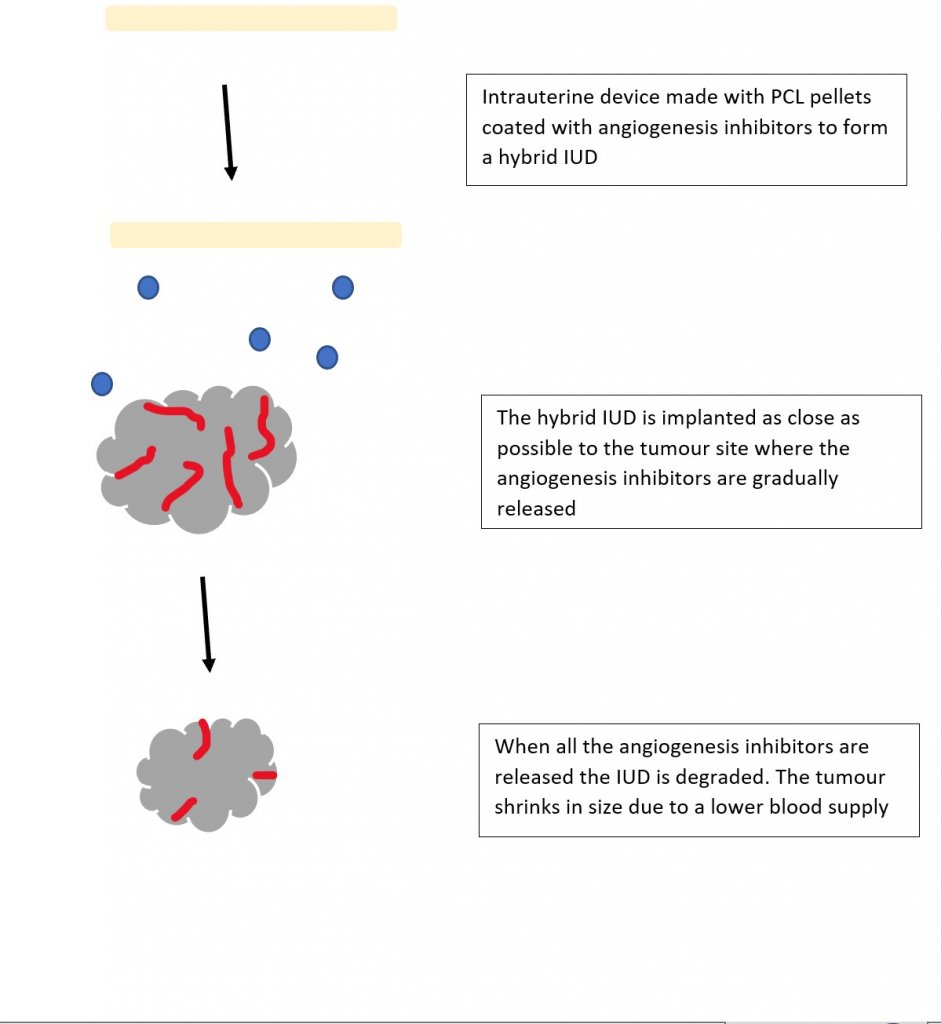
I am very familiar with the growth factor VEGF and its role in bone vasculature, so I really enjoyed (Poldervaart et al., 2014) paper on designing gelatin microparticles which had a controlled release of VEGF. Migration assays showed that the VEGF release from these microparticles lasted up to 3 weeks and was able to induce migration on endothelial progenitor cells. I began to wonder if 3D printing could be used for the potential treatment of bone cancer. What if we combined the biodegradable IUD concept but instead of hormones it contained angiogenesis inhibitors?
Organoids and chip in organs
To test my IUD hybrid idea, I could also utilise bioprinting to create organoids which are tiny 3D tissue culture that allows us to test the effects of infectious diseases and drug therapies. Organoids are rarely studied alone and are often in association with chip-in-organs, which replicate the organ environment so the results are more accurate to the behaviours that would be seen within the body.
Future Prospects
A lot of this research is very new and perhaps the two most obvious next steps for Bioprinting are refining the bioprinting mechanism itself and clinical trials, which some predict could occur within the decade. Most importantly, the ethical side of this needs to be discussed. Currently the FDA does not have an official definition for bioprinting, making it harder to set laws and guidelines for it. There is also a bit of worry about potential ‘black market’ uses of these resources – where do we draw the line? I could imagine that people could use this for recreational drugs such as nicotine for example or body builders could use it to increase their testosterone levels
However, the bioprinting world is such a new concept in the world of science with very promising results that could completely change the way we use therapeutics entirely and to be honest I am very excited to witness this.