The lectures I recently received about tissue engineering piqued my interest, specifically with the commercial availability of autogenic cells such as CarticelTM and as I looked further the development of such products was very interesting.
The 4 generations of autogenic chondrocyte implantation (ACI):
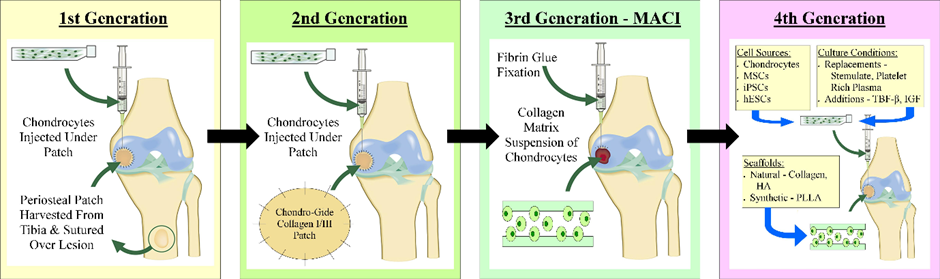
First Generation:
A 1st generation ACI procedure is shown in the video below where you can see the harvesting of periosteal tissue from the tibia, suturing of the periosteum into the knee joint, securing with fibrin glue and finally the injection of chondrocytes below this periosteal patch. Genzyme is a company which delivers this service and has reported success rates of 70-90%. Problems with this procedure include overgrowth of the implanted cells which can degrade joint function and cause pain; however this can be easily fixed by the shaving away of excess cartilage. Procedures of this type cost around $40,000, far too expensive for many people, especially in countries without nationalised healthcare such as America where insurance may not cover the procedure.
Second Generation:
Carticel is an example of 2nd generation ACI. A biopsy of cartilage is taken from lesser weight bearing areas so that chondrocyte cells can be isolated and expanded over a period of 4-6 weeks. The expanded cells are reinserted into the damaged joint to form new, healthy cartilage. On their website, Carticel states that their product is intended for the repair of “symptomatic cartilage defects of the femoral condyle caused by acute or repetitive trauma, in patients who have had an inadequate response to a prior arthroscopic or other surgical procedure”. According to the Bioinformant, Carticel autologous chondrocyte implantation costs between $15,000 and $35,000. This cost raises ethical questions because a large subset of people who would benefit from this procedure cannot afford it.
Third Generation:
Spherox is a company which offers 3rd generation ACI with a £10,000 price tag, however the Royal Orthopaedic Hospital (ROH) in Birmingham has provided this procedure and it is now eligible for patients on the NHS according to the ROH website. Spherox works in a different way to Carticel, by taking chondrocytes and producing spheroids of neocartilage composed of expanded autologous chondrocytes and their associated matrix. A sample of healthy tissue is taken from the patient in keyhole surgery and the sample is grown into chondrocyte spheroids. When the spheroids are implanted into the patient’s knee cartilage, they bind to the defective tissue and produce new cartilage tissue. For NHS patients in the UK, Spherox has far fewer ethical concerns regarding cost because the price of the operation is less than the cost caused by such injuries if left untreated to both the NHS and the patient’s quality of life.
The Future:
4th generation ACI therapy has not yet entered mainstream medicine, however various trials are underway. Some research is investigating the role of gene therapy in cartilage repair producing “temporarily and spatially defined delivery of therapeutic molecules to sites of cartilage damage”. According to this paper, the use of elastin as a scaffold is being investigated, as well as the use of a nonviral gene delivery system to allow mesenchymal stem cells to produce osteogenic growth factors.
This is an initially reflective and well researched blog showing how you have chosen to explore the emerging field of cartilage tissue engineering/cell therapy. You explore relevant information and arrange it logically in a manner that makes it easy for the reader to grasp.
You could improve by building in reflection throughout your blog (not just a the beginning) and by taking a slightly more critical approach – although your arrange and report the various technologies, you do not compare or contrast them, or weight up their qualities vs weaknesses.