Would it even be natural for a human to regrow a limb?
When we think of animals capable of regenerating lost body parts, amphibians like salamanders and axolotls are the first to come to mind. These creatures can regrow entire limbs, a process that humans are not naturally capable of—at least not in the same way. While humans can regenerate certain tissues (like liver and skin), regrowing complex structures like limbs remains beyond our biological abilities. However, scientific research is uncovering ways to potentially change that.
The Regenerative Powers of Amphibians
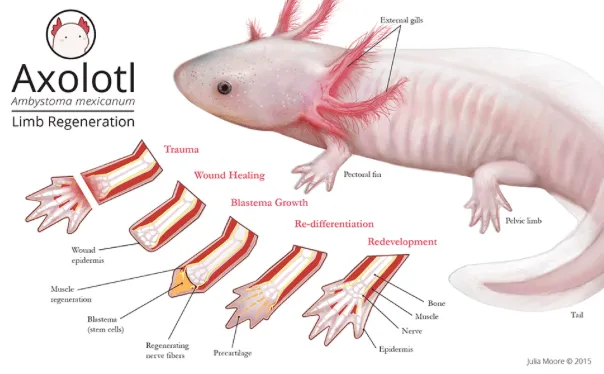
Amphibians, particularly species like axolotls (Ambystoma mexicanum) and salamanders (like Pleurodeles waltl), are famous for their regenerative abilities. When they lose a limb, they don’t just heal—they regrow the entire structure, including bones, muscles, nerves, and skin. This process begins with the formation of a blastema, a mass of undifferentiated cells at the injury site. These cells revert to a more stem-cell-like state and have the potential to differentiate into all the required tissues, such as bones, muscles, and nerves, with specific types of stem cell-like cells at localized areas of the body.
The regeneration process in amphibians is regulated by specific genes and molecular pathways. One of the key players in limb regeneration is FGF8 (Fibroblast Growth Factor 8), which promotes blastema formation and tissue growth (Liu et al., 2017). Additionally, GDF11, a regenerative gene, has been shown to play a role in promoting limb regeneration by controlling stem cell activity and reprogramming cells (Blum et al., 2019). Wnt signaling is another pathway that controls the proliferation and differentiation of these regenerative cells.
Why Can’t Humans Grow Limbs?
Humans, unfortunately, lack the regenerative powers of amphibians. When humans experience an amputation or injury, the body’s primary response is to form scar tissue. Scar tissue helps seal the wound but does not regenerate functional tissues like bones or muscles. Unlike amphibians, humans cannot activate the cellular mechanisms necessary for full limb regeneration. Although humans do have some regenerative abilities, such as the regeneration of skin or liver tissue (compensatory hyperplasia), these processes are much more limited and typically don’t extend to complex structures like limbs, instead simply multiplying the structures to restore the mass rather than creating various structures and mechanisms.
Evolutionarily, mammals have prioritized quick wound healing and survival over limb regeneration. The regenerative pathways seen in amphibians are not active in humans, and while we can regenerate simple tissues, regrowing complex structures such as limbs requires a much more intricate series of cellular events that humans are not biologically equipped to trigger.
The Search for Limb Regrowth in Humans
That said, researchers are not giving up. One exciting area of study involves the Lin28a gene, which plays a key role in cellular reprogramming. In amphibians like axolotls, Lin28a is activated during the early stages of regeneration and helps cells revert to a more flexible, regenerative state and is partially why axolotls retain infant features into adulthood. When Lin28a is activated in mammalian cells, it has been shown to promote reprogramming and regeneration (Zhou et al., 2018). Scientists are investigating whether activating this gene in humans could kick-start the regenerative process.
Another area of focus is stem cell technology (which I highlighted in my previous blog post as a central focus for me in this module). Stem cells are pluripotent, meaning they can differentiate into many types of cells. Scientists are exploring how to use stem cells, along with gene editing technologies like CRISPR-Cas9, to stimulate regeneration in damaged tissues. The hope is that by activating specific genes, such as Lin28a or Sox2, scientists might be able to push human cells into a regenerative state similar to that seen in amphibians (Takahashi & Yamanaka, 2006).
Would It Be ‘Natural’ for Humans to Regrow Limbs?
The question I now wish to ponder on, is whether it would be “natural” for humans to regrow limbs. While humans do not currently possess the same regenerative abilities as amphibians, nature has already demonstrated that limb regeneration is possible. If species like axolotls can do it, why not humans? Human evolution may not have favored limb regeneration, but there’s nothing inherently unnatural about the process if it can be achieved through genetic and stem cell technologies.
In the end, whether limb regeneration is “natural” might depend on one’s perspective. If it offers a chance to restore function and quality of life, it could be seen as a positive step forward for humanity—much like other medical breakthroughs that have altered the course of human health.
References:
- Blum, J. J., et al. (2019). “Regeneration in axolotls: Mechanisms and applications.” Journal of Experimental Biology, 222(14), jeb204645. DOI: 10.1242/jeb.204645
- Liu, J., et al. (2017). “Fibroblast growth factor 8 and limb regeneration.” Nature Communications, 8, 1313. DOI: 10.1038/s41467-017-01468-9
- Zhou, J., et al. (2018). “Activation of Lin28a gene in zebrafish restores regenerative potential.” Nature Biotechnology, 36, 452–459. DOI: 10.1038/nbt.4145
- Takahashi, K., & Yamanaka, S. (2006). “Induction of pluripotent stem cells from mouse fibroblasts by defined factors.” Cell, 126(4), 663–676. DOI: 10.1016/j.cell.2006.07.024
This is a good blog. It nicely demonstrates a good understanding of organ-on-a-chip technology and clearly explains its purpose and…
This is a good blog, very engaging with a good backgroud to 3D bioprinting. You could improve your blog with…
This is a good, very interesting blog about necrobotics. It explores the idea of necrobiotics which is fairly new approach…
This is a good blog. You introduce the reader to the topic of prosthetics and bionic limbs in a very…
This is a good blog introducing hernia mesh benefits and drawbacks. You create a narrative in this blog, which showcase…