Spinal chord injuries (SCIs) due to trauma or disease can have a profound impact on a persons life. Damage to the delicate nerves within the spinal chord can disrupt signals from the brain to the muscles which can result in loss of sensation or paralysis. As an avid rock climber, injuries such as SCIs can be a devastating reality. My bodies ability to function is integral to the sport I love. I don’t know what I would do without it.
Treatment options for SCIS are often limited and uncertain. Many have to come to terms with their injury and learn to adapt to it. But what if they didn’t have to? Stem cells have the remarkable ability to turn into any type of cell, including nerve cells! The idea is simple – why don’t we use artificially grown nerve cells to replace damaged cells?
Early studies have show that stem cells can promote nerve regeneration and restore limited function in SCIs. Watch this video to hear Chris’s story!
How can stem cells be used?
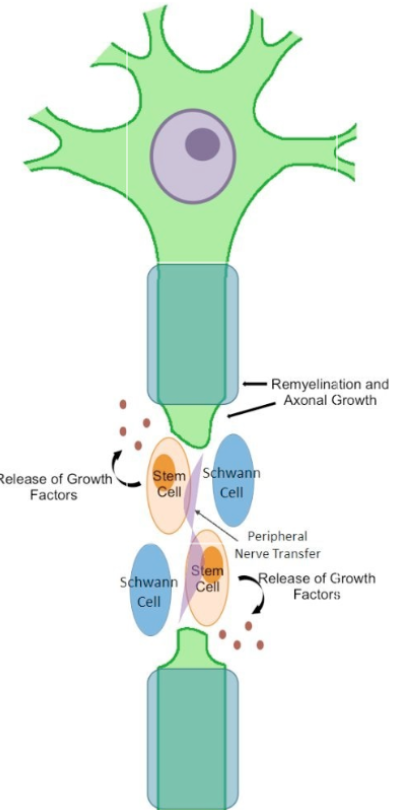
Abilities to repair damaged nerves with stem cells is currently limited but promising! The nervous system especially after trauma, is complex and unfortunately struggles to heal itself.
Here’s what stem cells can help with:
- Regenerate new nerve cells
- Release growth factors and proteins to protect damaged nerves.
- Promote mature stem cells to grow and reconnect.
- Break down scars at the site of damage enhancing growth.
This video explains it in more detail!
Stem cells can help promote nerve growth and repair, to reconnect the pathways and restore movement. This is slowly redefining what we think irreparable damage is.
Challenges that remain
Research is slow due to a high level of regulation around the gathering of embryonic stem cells. This undoubtedly hinders research that could have profound changes on someone’s life. Initially I saw these restrictions as an annoyance to scientific advancements, however discussions highlighted concerns of exploitation, destruction of potential life and wider public perception and trust. These views left me conflicted. Imagine if it was your ability to walk that hung on the line?
There are also concerns over whether the risk of stem cells out-way potential rewards which raises certain moral/ ethical questions.
| Risk | Moral/ethical dilemma |
| The extent of meaningful functional recovery is currently limited and uncertain. | Is it worth going through an operation to potentially only regain slight tingling? |
| Stem cells have a high likelihood of forming tumour cells. | Would you want to risk cancer? |
| Immune system might reject and attack the new stem cells. | Surely attempting something that might be beneficial is better than not doing anything? |
This entire time I’ve been assuming that people want to repair nerve damage. There has been pushback in the deaf community surrounding the use of cochlear implants as some believe there’s no need to ‘fix’ those hard of hearing. Its possible that people with SCIs, especially those born with the condition might not even want ‘normal’ function. Would you want to change yourself again/ for the first time after already becoming comfortable and able in your body? I’m not so sure that I would.
Nerve damage repair with stem cells holds promise but there sadly there are no proven treatments that exist yet. I cant wait to see the progress and advancements that become availible over the next few decades. Do you think SCI repair will be possible someday? I hope so.
Answers to your questions about stem cell research (no date) Mayo Clinic. Available at: https://www.mayoclinic.org/tests-procedures/bone-marrow-transplant/in-depth/stem-cells/art-20048117 (Accessed: 28 March 2025).
Assen, L.S. et al. (2021) ‘Recognizing the ethical implications of stem cell research: A call for broadening the scope’, Stem Cell Reports, 16(7), pp. 1656–1661. Available at: https://doi.org/10.1016/j.stemcr.2021.05.021.
Figure 2. Schematic representation of the repair of peripheral nerve… (no date) ResearchGate. Available at: https://www.researchgate.net/figure/Schematic-representation-of-the-repair-of-peripheral-nerve-damage-with-a-stem-cell-based_fig1_341051575 (Accessed: 25 March 2025).
Lavender, F. (2021) ‘The stigma around cochlear implants – Deaf Action’, 30 September. Available at: https://deafaction.org/ceo-blog/the-stigma-around-cochlear-implants/ (Accessed: 28 March 2025).
New hope for spinal cord injuries (2024). Available at: https://www.youtube.com/watch?v=D_GuSZT_6eI (Accessed: 28 March 2025).
Progress and Promise of Stem Cell Research: Fixing nerve damage in spinal cord injury (2017). Available at: https://www.youtube.com/watch?v=QdBW4ntaimc (Accessed: 28 March 2025).
Stem Cell Research Controversy: A Deep Dive (2024) (no date). Available at: https://www.dvcstem.com/post/stem-cell-research-controversy (Accessed: 28 March 2025).
Sullivan, R. et al. (2016) ‘Peripheral Nerve Injury: Stem Cell Therapy and Peripheral Nerve Transfer’, International Journal of Molecular Sciences, 17(12), p. 2101. Available at: https://doi.org/10.3390/ijms17122101.







This is a good blog. It nicely demonstrates a good understanding of organ-on-a-chip technology and clearly explains its purpose and…
This is a good blog, very engaging with a good backgroud to 3D bioprinting. You could improve your blog with…
This is a good, very interesting blog about necrobotics. It explores the idea of necrobiotics which is fairly new approach…
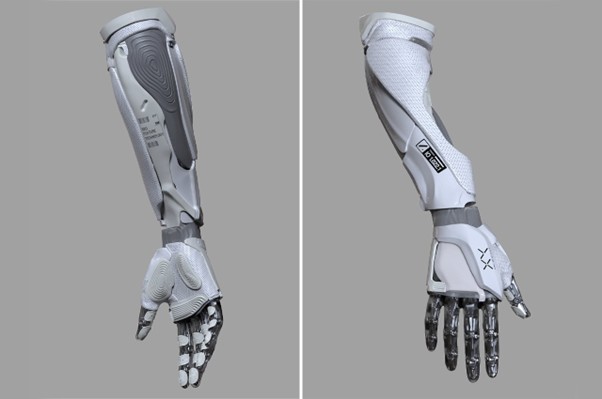
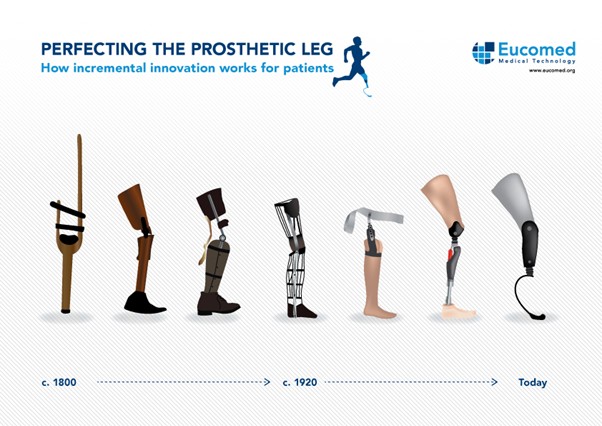
This is a good blog. You introduce the reader to the topic of prosthetics and bionic limbs in a very…
This is a good blog introducing hernia mesh benefits and drawbacks. You create a narrative in this blog, which showcase…