As medicine becomes increasingly tailored to individual needs, the one-size-fits-all approach is fading into the past. Thanks to advancements in stem-cell technology and tissue engineering, doctors are developing personalised treatments, designed for each patient. Among these breakthroughs, induced pluripotent stem cells (iPSCs) are pushing the boundaries of personalised medicine, offering therapies tailored to our unique genetic makeup. However, as this vision becomes a reality, critical questions emerge: how far can we take personalised medicine, and what hurdles stand in the way?
Stem cells in personalised medicine
Stem cells have a unique ability to self-renew and differentiate into various cells, making them invaluable in disease modelling and regenerative medicine. iPSCs, created by reprogramming adult cells to behave like embryonic stem cells (ESCs), offer a powerful tool in personalised medicine. As they are crafted from a patient’s own cells, iPSCs enable highly individualised therapies:
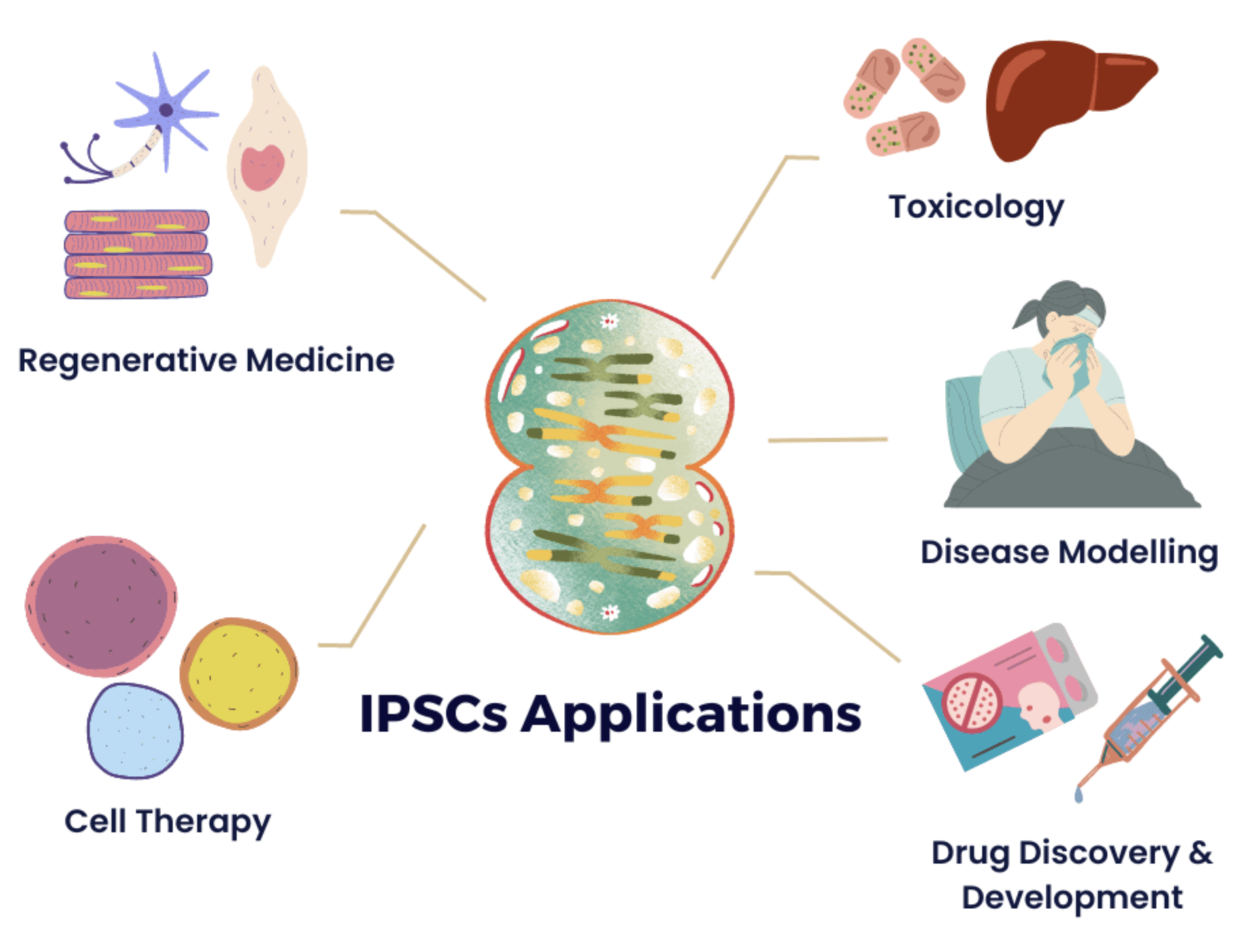
- Disease modelling: iPSCs allow researches to create patient-specific cell models to study diseases and test targeted treatments
- Personalised drug testing: scientists can predict how a patient will respond to specific drugs, minimising trial-and-error in prescribing medications
- Regenerative medicine: iPSCs can generate patient-specific tissues for transplants, reducing immune rejection and improving long-term success
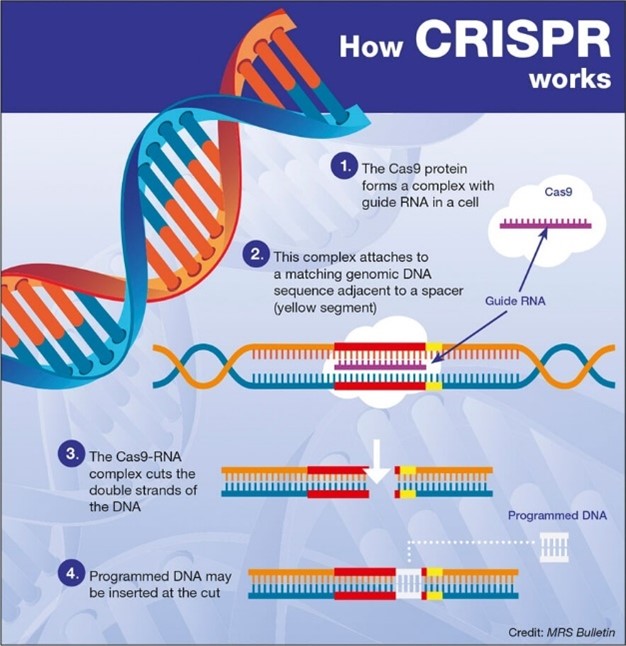
- Gene editing: iPSCs can be genetically modified (e.g. CRISPR) to correct mutations, offering potential cures for genetic diseases
Advantages of iPSCs over past technologies:
iPSCs over embryonic stem cells
Unlike ESCs, iPSCs bypass the ethical concerns of destroying human embryos, making them a more widely accepted alternative, particularly in areas with stricter bioethical regulations. Since they are derived from the patient’s own cells, iPSCs are genetically identical enabling patient-specific disease modelling and reducing the risk of immune rejection – a major concern with ESCs.
iPSCs over animal models
iPSCs offer a more accurate representation of human disease, improving relevance and predictability of experimental outcomes, as physiological and genetic differences between species can give rise to misleading results in research. iPSCs also bypass ethical concerns in animal testing, allowing a more humane alternative approach to research and drug testing. Additionally, iPSCs rapidly proliferate in culture, allowing high-throughput screening that is difficult to achieve with animal models.
Balancing innovation and ethics
Whilst iPSCs evade the ethical dilemmas of past technologies, concerns remain:
- Privacy: iPSCs contain sensitive genetic data that could be misused
- Genetic manipulation: gene editing technologies may be exploited for enhancing traits rather than treating diseases
- Inequality: the high cost of iPSC therapies could make them accessible only to the wealthy, deepening health disparities
The first success story

In 2014, Japan conducted the first clinical study using iPSCs. Masayo Takahashi led a groundbreaking trial transplanting iPSC-derived retinal pigment epithelial (RPE) cells to treat age-related macular degeneration. Whilst promising, high costs and lengthy cultivation times posed challenges. Scientists overcame this by developing allogenic iPSCs from rare donors, making iPSC therapy more accessible. In 2017, five successful allogenic RPE transplants were performed by Kobe City Medical Centre and Osaka University [1].
Looking ahead
iPSCs offer unparalleled opportunities to redefine medicine, from regenerative therapies to truly personalised treatments. This marks a promising shift away from the traditional one-size-fits-all approach, paving the way for customised healthcare tailored to each individual. While challenges remain, continued research and innovation ensure that the future of personalised medicine is bright – and this is only the beginning!






This is a good blog. It nicely demonstrates a good understanding of organ-on-a-chip technology and clearly explains its purpose and…
This is a good blog, very engaging with a good backgroud to 3D bioprinting. You could improve your blog with…
This is a good, very interesting blog about necrobotics. It explores the idea of necrobiotics which is fairly new approach…
This is a good blog. You introduce the reader to the topic of prosthetics and bionic limbs in a very…
This is a good blog introducing hernia mesh benefits and drawbacks. You create a narrative in this blog, which showcase…