Growing up, I was fascinated by the stories of superheroes — beings with enhanced strength, rapid healing, or minds that could outthink any machine. As part of my school project, I stumbled upon the concept of nanobots, and realised the extraordinary powers I always admired weren’t just confined to comic books. The idea of microscopic machines navigating the body, targeting diseases with precision and sparing healthy cells, felt like a real-life superpower. Suddenly, the thought of humans enhanced by technology didn’t seem so impossible. What if we could repair injuries in seconds, regenerate damaged tissues, or even enhance our mental and physical abilities? Welcome to the world of nanotechnology, where the future isn’t merely bigger; it’s smaller, smarter, and infinitely more extraordinary.
An Overview of the Nanoscale World
Nanotechnology, the manipulation of matter on an atomic and molecular scale, has become a ground-breaking force across various fields. Nanoparticles, typically measuring between 1-100 nanometres, possess unique properties that offer transformative potential. From medicine to environmental solutions, nanotechnology has rapidly advanced, with estimates predicting a global worth of over $33.63 billion by 2030. Among its most promising applications are nanobots — microscopic robots capable of performing intricate tasks at the cellular level.
Nanobots excel in diagnostics by detecting disease biomarkers before symptoms arise. Embedded with nanosensors in the bloodstream, they provide real-time health data, enabling early warnings for conditions like cancer, Alzheimer’s, or cardiovascular disease. Could nanobots be the answer to cure incurable diseases?
The Birth of the Superhuman
Nanobots are no longer just medical marvels; they may become the gateway to the next phase of human evolution. With nanobots running through our veins, the boundaries of human capabilities could blur, turning science fiction into reality. The superhuman, once confined to comic books, might walk among us — with unparalleled strength and augmented intelligence. From curing genetic disorders to amplifying memory and endurance, nanotechnology might redefine man.
Moreover, the superhuman is not solely about individual enhancement — nanobots could make collective intelligence a reality. By linking humans into a shared neural network, nanotechnology could foster a “hive mind” where ideas, skills, and knowledge are exchanged instantaneously. Imagine a world where a surgeon’s precision, an artist’s creativity, or a scientist’s discoveries can be instantly shared and applied by anyone connected to the network. This shared intelligence would accelerate evolution and drive progress at a pace beyond anything we’ve ever imagined.
The Price of Evolution
The prospect of engineered enhancement raises profound ethical questions. Who gets access to these upgrades, and will they deepen the divide between privileged and disadvantaged? Wealthier individuals could prolong their lives, enhance cognitive abilities, or prevent diseases before they emerge. Meanwhile, marginalised communities might be left behind. Will we see the dawn of a new medical aristocracy, where longevity and vitality are privileges of the wealthy?
Merging Man and Machine
With nanobot-driven evolution, we may face dilemmas even beyond economic disparity. The line between self and machine could blur to the point where human identity itself is called into question. Are we still human if a significant portion of our bodies and minds are machine-optimised? Could the ability to alter emotions and memories undermine the very fabric of personal identity?
Furthermore, the concept of mortality may shift. With nanobots repairing cells, reversing damage, and potentially halting aging, the natural cycle of life and death could be disrupted. While the idea of a prolonged, healthier life is desirable, it might force us to reconsider our relationship with time, purpose, and the meaning of existence.

References
Haleem, A., Javaid, M., Singh, R.P., Rab, S. and Suman, R. (2023). Applications of Nanotechnology in Medical field. Global Health Journal, [online] 7(2). doi:https://doi.org/10.1016/j.glohj.2023.02.008.
Hypothetica (2024). Nanobots – The next chapter in human evolution. [online] Substack.com. Available at: https://hypothetica.substack.com/p/nanobots-the-next-chapter-in-human [Accessed 28 Mar. 2025].
Moore, S. (2021). An Overview of Nanobots and the Most Recent Developments. [online] AZoNano.com. Available at: https://www.azonano.com/article.aspx?ArticleID=5761.
Vrilya Jarac (2023). Beyond Boundaries: The Nanobot Revolution and the Future of Human Augmentation. [online] Medium. Available at: https://vrilyajarac.medium.com/beyond-boundaries-the-nanobot-revolution-and-the-future-of-human-augmentation-3975e35ed599 [Accessed 28 Mar. 2025].

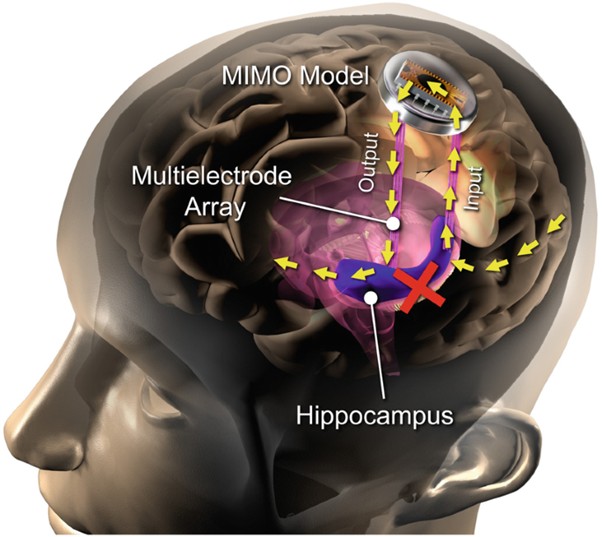
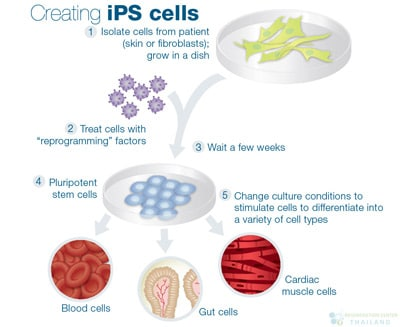
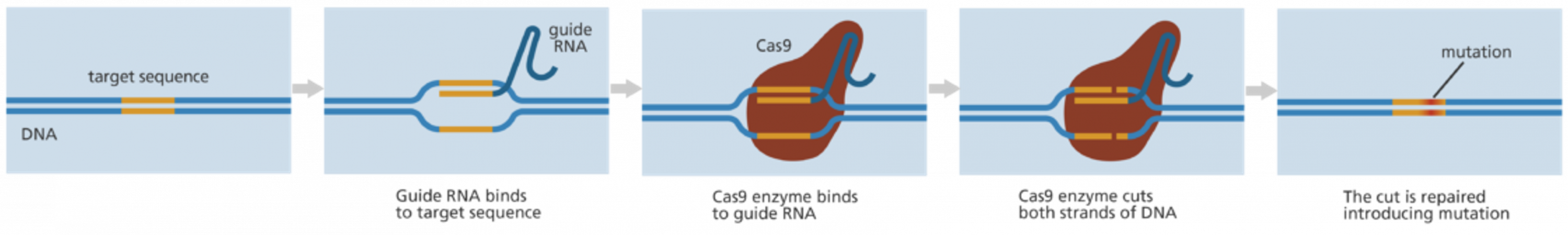


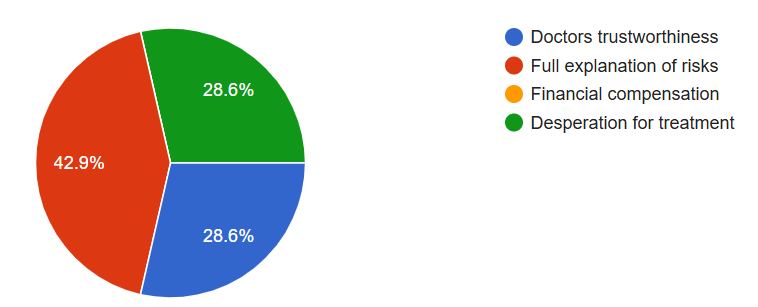




This is a good blog. It nicely demonstrates a good understanding of organ-on-a-chip technology and clearly explains its purpose and…
This is a good blog, very engaging with a good backgroud to 3D bioprinting. You could improve your blog with…
This is a good, very interesting blog about necrobotics. It explores the idea of necrobiotics which is fairly new approach…
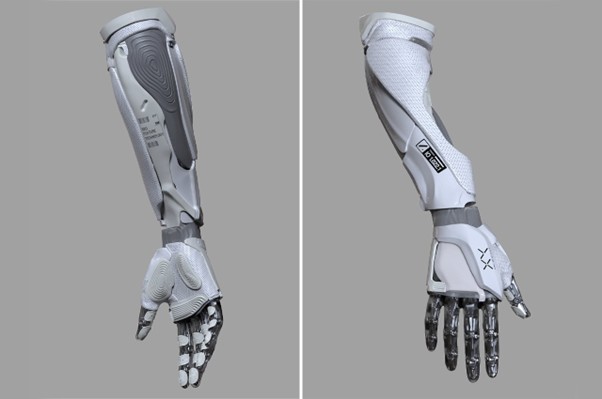
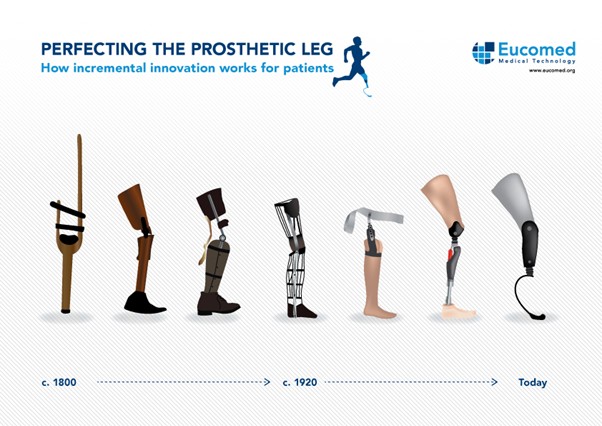
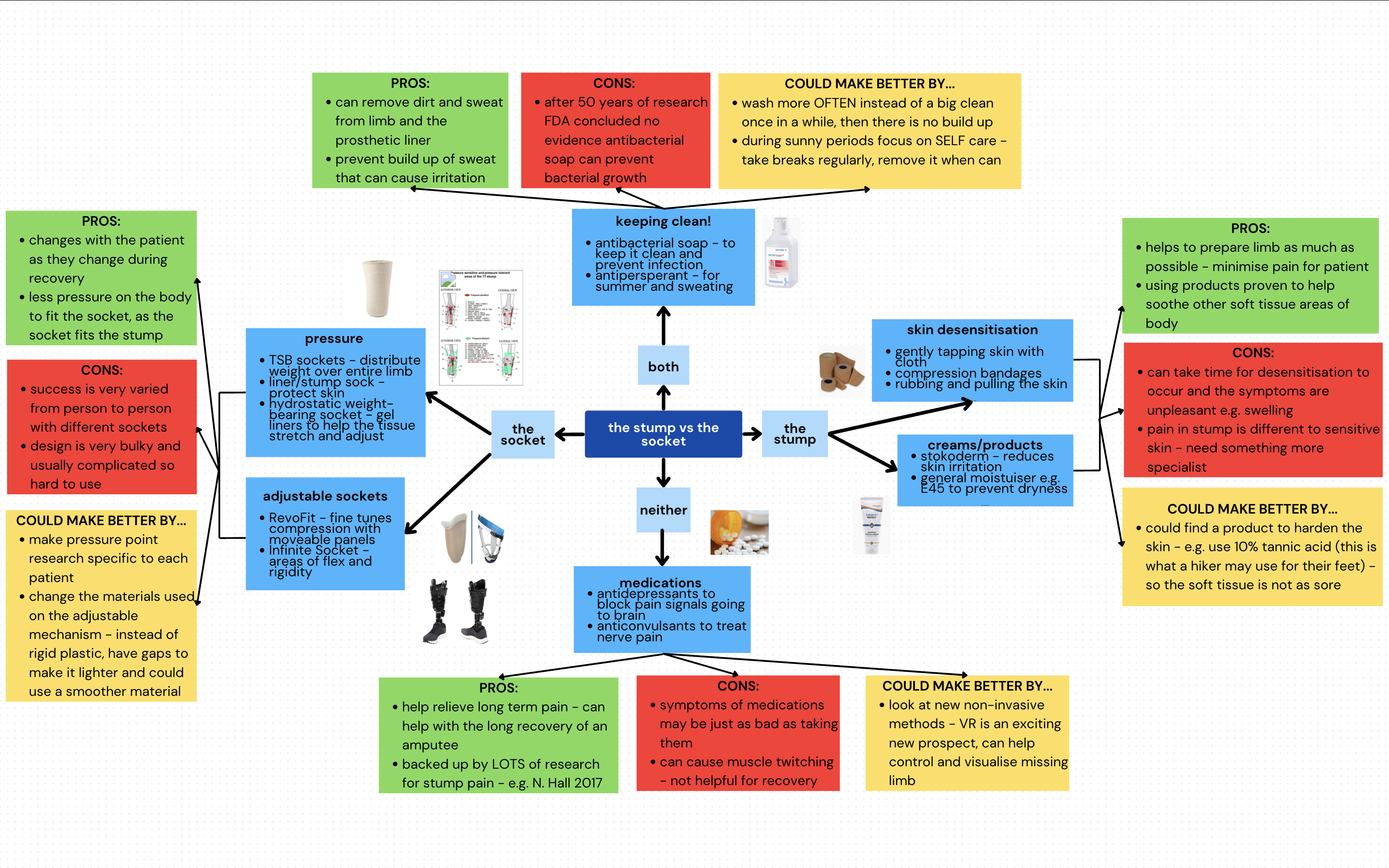
This is a good blog. You introduce the reader to the topic of prosthetics and bionic limbs in a very…
This is a good blog introducing hernia mesh benefits and drawbacks. You create a narrative in this blog, which showcase…