Imagine if your ability to eat relied on whether you could solve a handful of maths questions, lines of algebra blocking you from simply reaching over to the snack drawer and grabbing a bag of your favourite crisps. Sounds like a nightmare, right? But for 464,000 people in the UK alone, this is a version of their reality whilst living with type 1 diabetes, an autoimmune disease that prevents your body from producing insulin [1].
What is insulin?
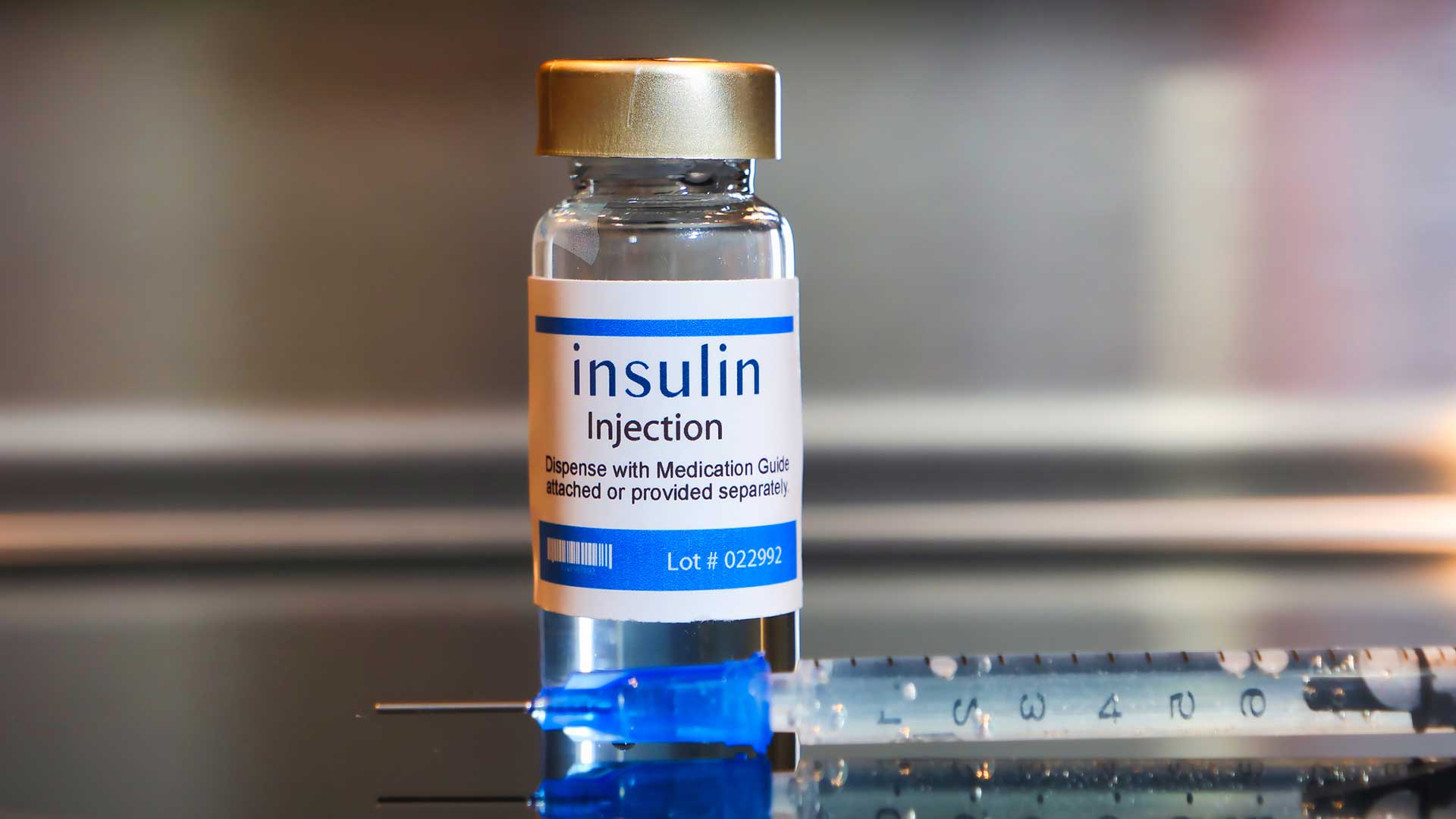
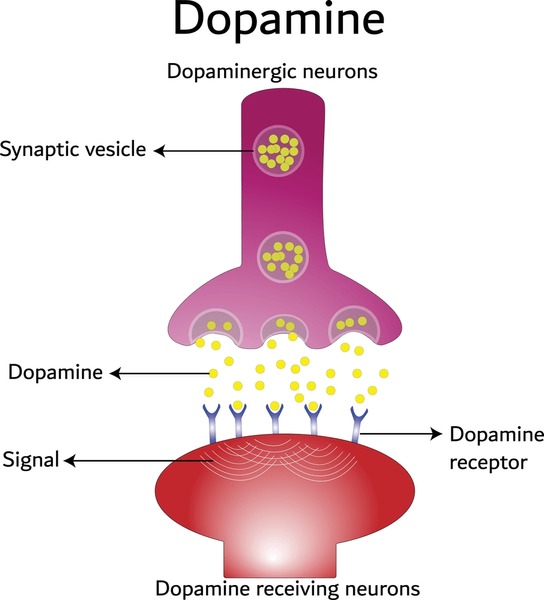
Insulin, a vital hormone produced by the pancreas after the ingestion of food, enables our body to transfer glucose from our blood into our cells, allowing it to be used as energy [2].
Image available at: https://gluroo.com/blog/diabetes-101/insulin-faqs-type-1-diabetes/ (accessed: 09/03/25)
Those with type 1 diabetes have a pancreas that is unable to produce enough insulin to promote this transfer causing their levels of blood glucose to slowly increase with each meal, the sugars predominantly being expelled only as waste via frequent urination. As a result, insulin must be supplemented through injection, quantities being meticulously calculated dependent on what is being eaten and when. Whilst with years of practice calculating the amount of insulin you need can become almost second nature, many with this disease long for a day where they can eat freely, not having to constantly monitor their blood sugar levels and symptoms.
A short video explaining some key aspects of type 1 diabetes – closed captions are available!
Current Technologies
Whilst technology currently exists which aims to make the lives of those with diabetes easier, nothing works quite as well as a pancreas. For example, DexCom is a company which produces a small sensor which allows for the continuous monitoring of blood sugar levels, a connecting app receiving and displaying the statistics on a regular basis. Having an error of only 8.2%, this sensor would appear to be amazing in almost any other application, but when it comes to someone’s health you can never be accurate enough [3].
This video follows a young woman called Jordan and how DexCom allowed her to regain control of her life and love for sport (closed captions available)
With pancreas transplants carrying the same risks as any other major surgery, the possibility of rejection urging healthcare professionals to encourage the majority to manage their condition through diet and medication, most are left with these sensors as their most advanced form of care. Therefore, although some aspects of life with type 1 diabetes can be made more “convenient”, the fight for an accessible cure is still ongoing.
Stem Cells and Ethics
Excitingly, it has been reported that a 25-year-old woman with type 1 diabetes has undergone a stem cell transplant enabling her pancreas to begin producing insulin again, it continuing to do so even a year post transplant – more about this encouraging story can be read here! Whilst this seems promising, the procedure does come with risks. It is believed that a form of immunosuppression is required in order to prevent complications, making the patient more vulnerable to other illnesses and infections.
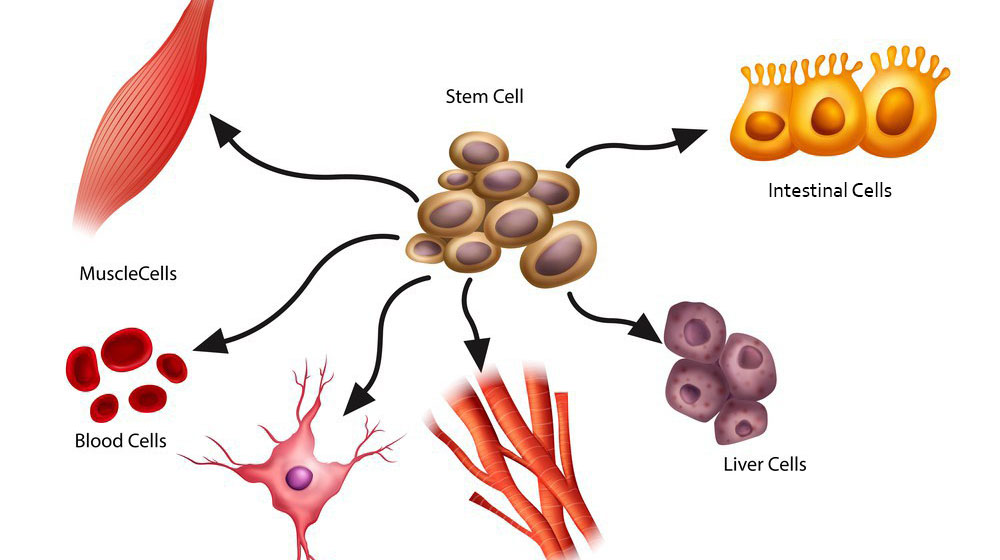
Furthermore, there is currently a large volume of debate over the ethics of using stem cells in treatments. Embryonic stem cells, those that are typically used in such treatments, can only be obtained through the destruction of a human embryo, a process that breeds concern surrounding the sanctity and rights of early human life [4].
Image available at: https://bioinformant.com/what-are-stem-cells/ (accessed: 09/03/25)
Whilst many believe using a potential human life in this manner is unethical, I can’t help but wonder if denying somebody a better quality of life that could be so easily provided is just as cruel. How would you feel if a cure to something that controlled your daily life was just out of reach?
References
[1]Diabetes UK, “How Many People in the UK Have diabetes?,” Diabetes UK, 2024. https://www.diabetes.org.uk/about-us/about-the-charity/our-strategy/statistics
[2] NHS , “What is type 1 diabetes?,” nhs.uk, Nov. 31, 2024. https://www.nhs.uk/conditions/type-1-diabetes/what-is-type-1-diabetes/
[3]S. K. Garg et al., “Accuracy and Safety of Dexcom G7 Continuous Glucose Monitoring in Adults with Diabetes,” Diabetes Technology & Therapeutics, vol. 24, no. 6, pp. 373–380, Jun. 2022, doi: https://doi.org/10.1089/dia.2022.0011.
[4] L. A. Cona, “Stem Cell Research Controversy: A Deep Dive (2023),” www.dvcstem.com, Sep. 14, 2023. https://www.dvcstem.com/post/stem-cell-research-controversy






This is a good blog. It nicely demonstrates a good understanding of organ-on-a-chip technology and clearly explains its purpose and…
This is a good blog, very engaging with a good backgroud to 3D bioprinting. You could improve your blog with…
This is a good, very interesting blog about necrobotics. It explores the idea of necrobiotics which is fairly new approach…
This is a good blog. You introduce the reader to the topic of prosthetics and bionic limbs in a very…
This is a good blog introducing hernia mesh benefits and drawbacks. You create a narrative in this blog, which showcase…