A brief introduction
One topic mentioned in the ethics workshop was egg donation. This is the practice of women donating their eggs either for IVF treatments or for scientific research. There is great variation in the payment of women in different cultures, sparking debate over how donors should be compensated.
How does the process work?
The process of egg collection is lengthy, taking two to three months. Genetic screening is also required before the egg collection occurs which follows a few key steps.
Firstly, hormone treatment occurs on day two of the cycle. This is done via daily FSH injections to boost the number of follicles formed. Secondly, after a few days of FSH injections antagonist injections occur to supress natural hormone production of the cycle. At this stage blood tests and scans are needed to check for responses to the medication. Further antagonist injections then help the eggs mature. Finally, to collect the eggs from the body pain relief via sedation or general anaesthetic may also be required. The collected eggs are then used fresh or are frozen for later use.

The UK law of egg donation
In the UK it is illegal to profit from this practice however compensation of £985 per cycle (one complete round of treatment) is allowed. This compensation is strictly processed based, not for actual donation and more can also be claimed to cover fees for expenses such as travel, accommodation and childcare.
Egg donation in the US
In the US the ASRM (American Society for Reproductive Medicine) requirements must be met, and it is possible to donate anonymously which is different to the UK law.
The cost of fresh egg donation ranges from $35, 000 to $50, 000 and is not always limited to processed based compensation, differing to UK law.
Ethical arguments in favour of payment
Egg donation is time consuming due to the duration travel and process. Daily injections also make it extremely inconvenient. There is also a risk to the health of the donor including risks associated with medication such as general anaesthetic and there is potential risk to life due to OHSS and side effects.
Counselling is also legally required, demonstrating the large mental health impact which can be long lasting and not fairly compensated for as mental health affects every aspect of life.
These reasons describe how it could be argued that there is not enough compensation for the risk taken by the donors.
Additionally, the US system assigns great value to the donated eggs which is reflected by the price women are paid for their eggs.
Ethical problems with payment for Egg donation
Major problems associated with paying for egg donation include encouragement to undergo a complicated and painful procedure for those that more urgently need money. A monetary incentive and other potential pressures could also introduce ethical issues about true consent which can be eliminated if the process is voluntary and unpaid.
Overall conclusions
It is a difficult topic with no complete solution. The current UK system allowing some compensation to cover the actual cost of donation feels the most appropriate to me as it offers a middle ground where the women are somewhat looked after but no moral compromises are made. However, a slight increase in the maximum compensation limit could better support the women.

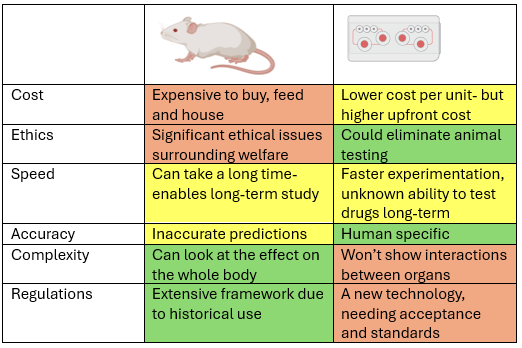
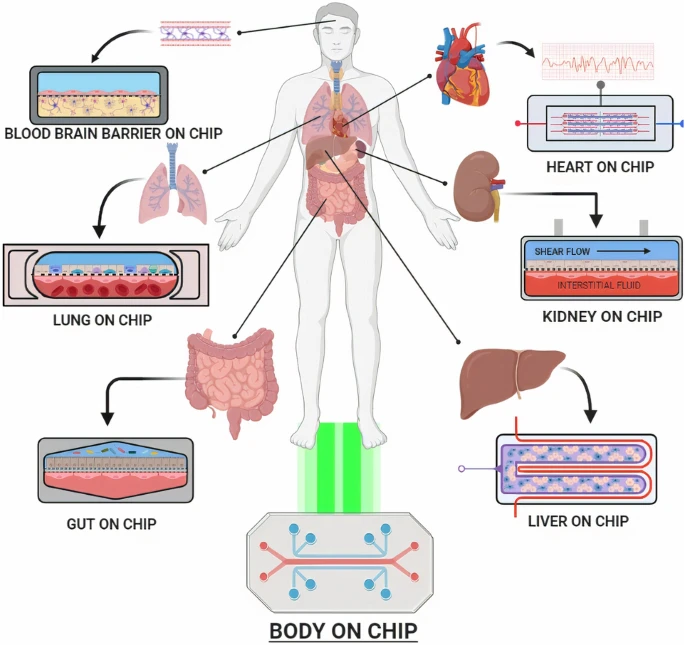


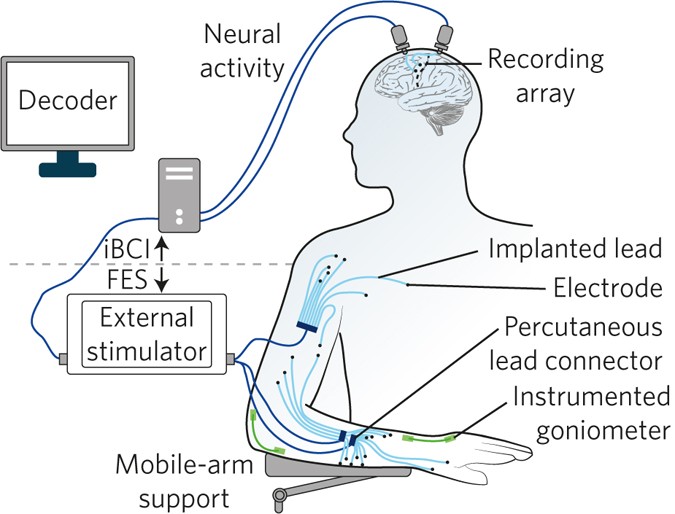

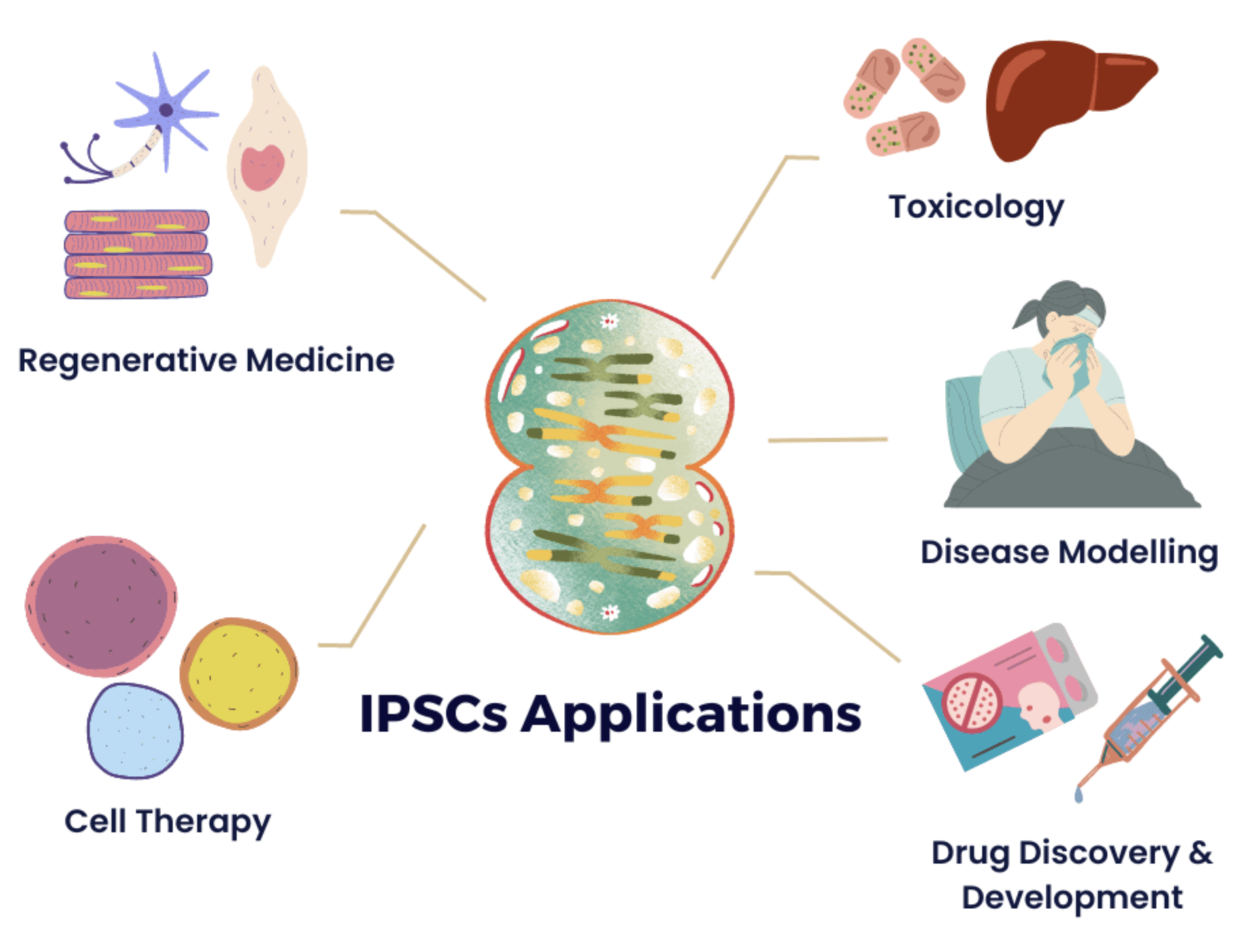


This is a good blog. It nicely demonstrates a good understanding of organ-on-a-chip technology and clearly explains its purpose and…
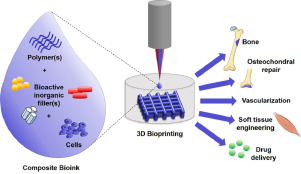
This is a good blog, very engaging with a good backgroud to 3D bioprinting. You could improve your blog with…
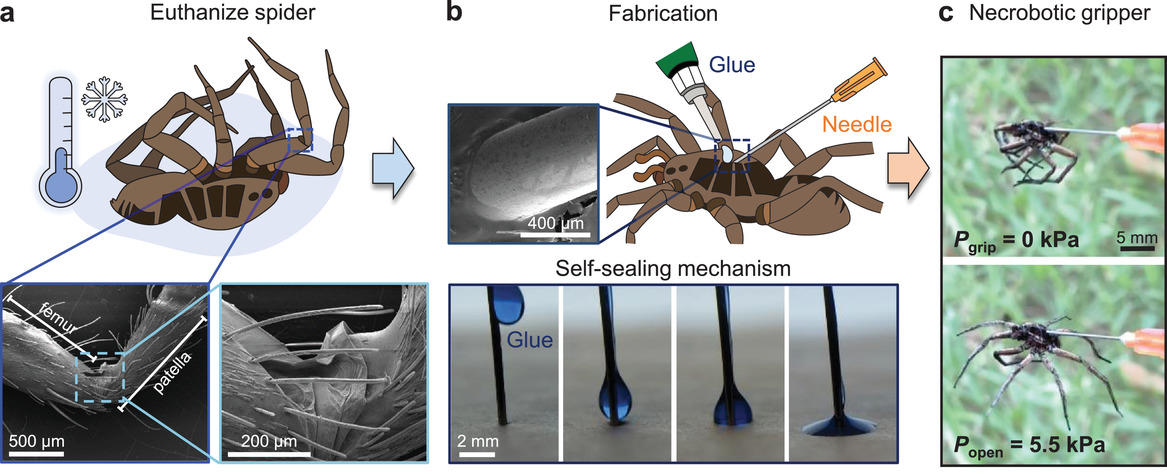
This is a good, very interesting blog about necrobotics. It explores the idea of necrobiotics which is fairly new approach…
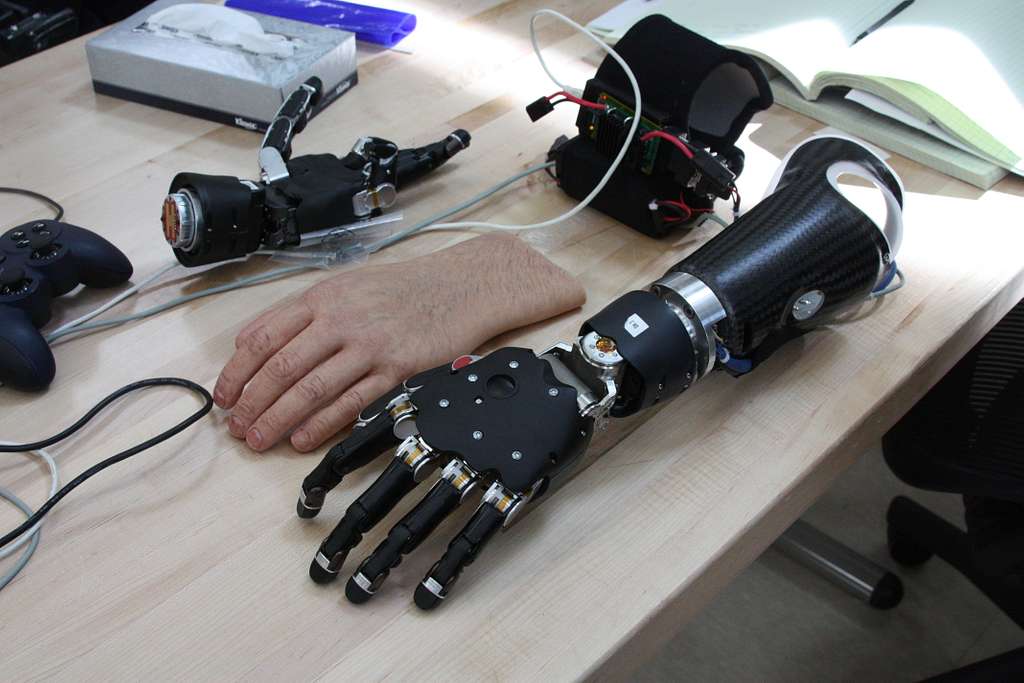
This is a good blog. You introduce the reader to the topic of prosthetics and bionic limbs in a very…
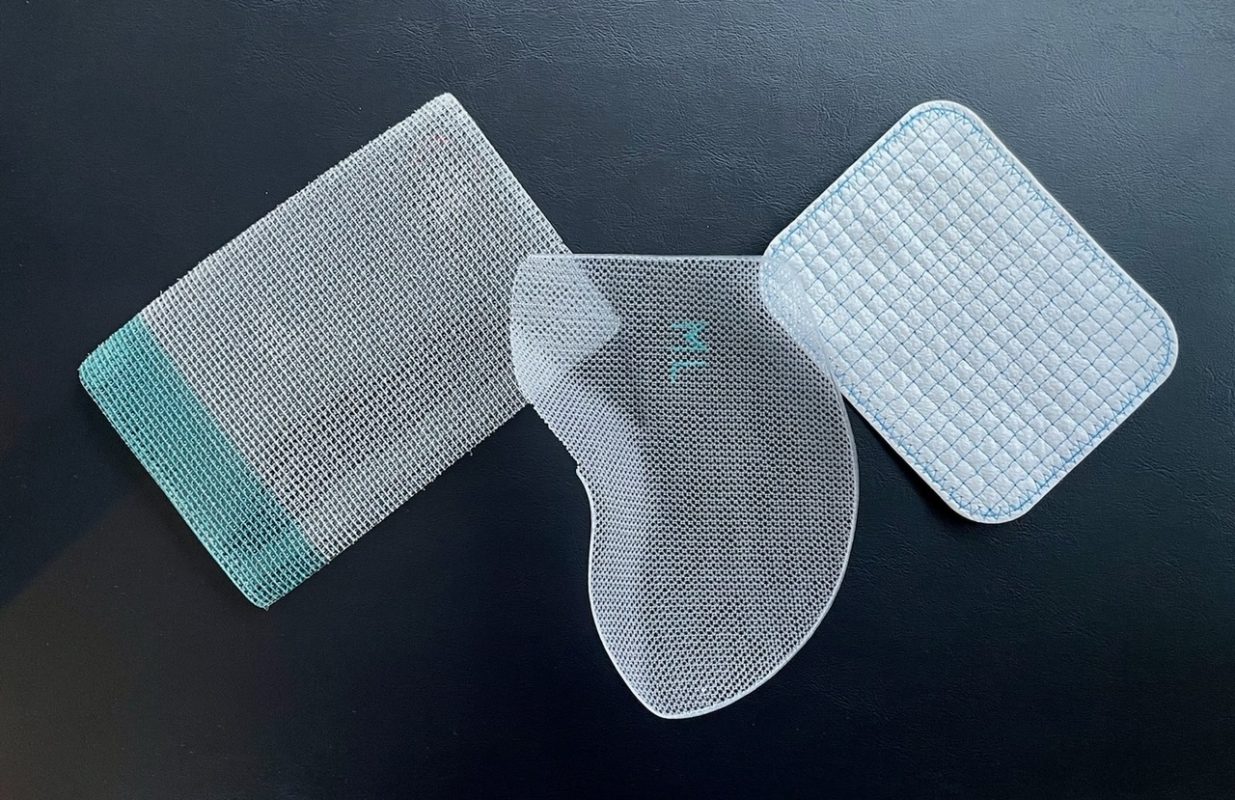
This is a good blog introducing hernia mesh benefits and drawbacks. You create a narrative in this blog, which showcase…