What if your doctor could test different treatments on a miniature replica of your organs before prescribing the perfect medication for you? This futuristic idea quickly becomes a reality with organ-on-chip (OoC) technology.
I first encountered OoCs when I was assigned my dissertation topic. Before that, I had never even heard of them. But as I delved deeper, the potential applications seemed limitless, from revolutionizing drug development to unexpected uses like measuring water pollution by simulating how a fish gill interacts with its environment (the focus of my research).
What are Organ-on-Chip Devices?
An organ-on-chip is a microfluidic device constructed to mimic the structure, microenvironment, and physiological functions of organs. These devices use microfluidic channels (small tubes) to transport essential nutrients, drugs, or toxins to a culture of living cells while removing any waste products. They vary in design depending on the organ they are simulating, offering a range of parameters like chemical gradients, mechanical shear, and tissue-tissue interactions making them ideal for drug screening and toxicity testing.
The video from Wyss Institute further illustrates how one of these innovative lung-on-chip devices works and its potential in the pharmaceutical field.
Source: Introduction to Organs-on-a-Chip
Why are Organ-on-Chip Devices Beneficial?
- Traditional 2D cell cultures cannot accurately reflect the structure, function, and mechanical behaviour of living tissue or its highly complex interactions with the rest of the body. OoCs bridge this gap by recreating key physiological conditions in a controlled microenvironment, offering better drug testing results than conventional methods.
- Developing a new drug is notoriously slow and expensive – it can take up to 10 years and cost more than $3 billion (that’s a lot of money!). This is mainly due to the reliance on animal testing before human trials. However, animal models have a high failure rate as their biological responses often do not always match human reactions. This leads to ineffective or toxic drugs progressing through clinical trials, while potentially effective compounds never moving to market.
OoCs address this issue by using human cells, making them more predictive of actual drug effects in people whilst being faster, cheaper, and requiring fewer resources. Allowing earlier detection of any harmful side effects earlier in development preventing costly late-stage failures. - Many people including myself consider OoCs a more ethical alternative to animal testing. By providing a more accurate alternative while minimizing reliance on animal testing.
Challenges of Organ-on-Chip Technology
- The current manufacture of OoCs is often performed in research labs requiring specialized fabrication techniques. Moving to mass production while maintaining its precision poses a significant challenge.
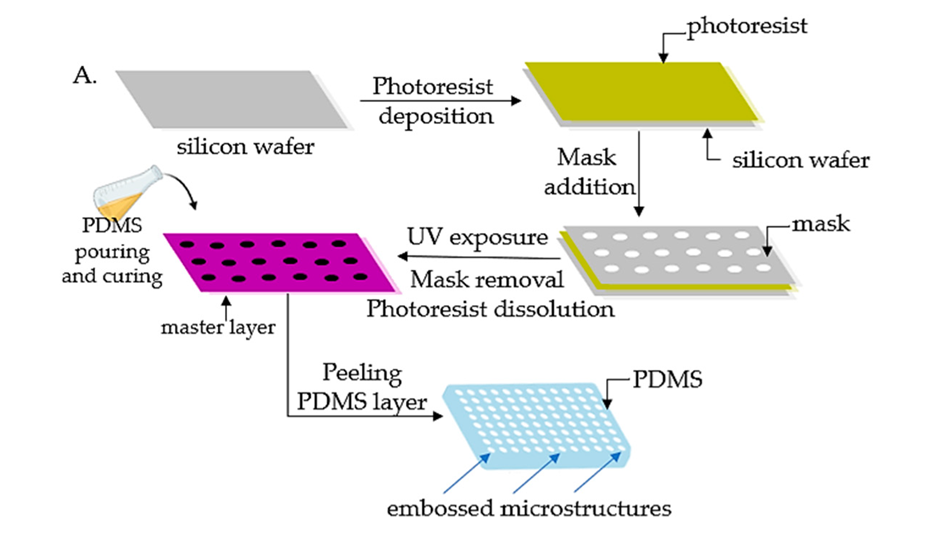
- Most OoCs use polydimethylsiloxane (PDMS) due to its biocompatibility, flexibility, and transparency properties to form microfluidic channels. However, PDMS absorbs small drug molecules, potentially affecting results. Finding alternative materials that do not interfere with drug testing is needed to improve accuracy.
Figure below providing further details on the fabrication process of an Organ on chip:
- OoCs are not perfect, they are simplified models of organs replicating only the key functions of the organ failing to capture the whole function and complex role within a human body.
- The use of human cells for OoCs still raises ethical questions, particularly regarding the sourcing of cells how will they be obtained are the doners consenting? OoC technology isn’t cheap limiting its benefits and availability across the world.
- There is a lengthy process before OoCs can be considered a suitable alternative to animal testing. This is governed by regulatory bodies like the FDA and EMA which require extensive validation.
- Long-term maintenance of cell cultures in OoC devices presents its own challenges, including ensuring a steady supply of nutrients, efficient removal of waste products, and maintaining stable microenvironmental factors such as pH and oxygen levels. Additionally, cellular senescence – the gradual loss of cells’ ability to divide and function further complicates its prolonged use.
The Future of OoCs Technology
OoC technology is rapidly advancing, with models already developed for organs including brain, gut, lung, heart, and more. However, there is a universal goal towards producing a human-on-chip. A human-on-chip integrates multiple OoCs into a single system, allowing simulation of organ-organ interactions within the body. This innovation would have applications in a multitude of areas:
- Personalized medicine: using a patient’s own miniaturised organ system to test various drugs to identify the most effective treatment.
- Drug development: providing a more effective approach by mimicking and tracking how the drugs travel through the entire body.
- Disease modelling: demonstrating how complex diseases like cancer or diabetes impact various organs over time, accelerating research and the discovery of effective treatments and cures.
The figure below further illustrates the human-on-chip system.
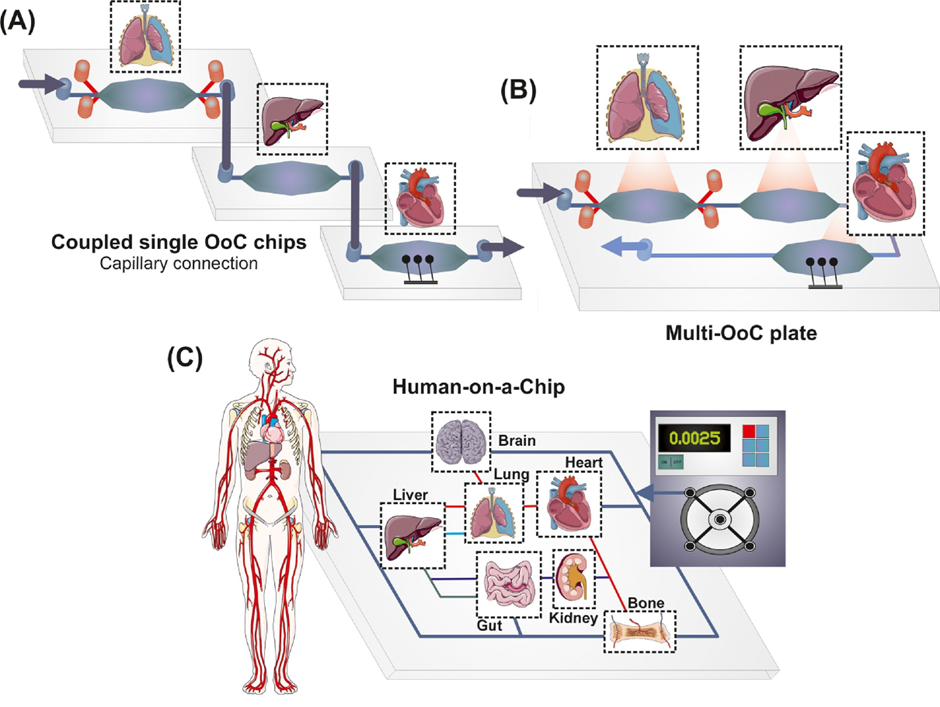
As I mentioned earlier, the potential of OoC technology is limitless and fascinating. The concept of a human-on-chip functioning as a “mini-me” to monitor health could revolutionise how we understand and maintain personal wellness. Imagine real-time updates about your body’s needs like nutrient deficiencies or predicting health issues before symptoms even appear.
While challenges remain, I am optimistic about their future and hope to see a wider implementation not just in medicine, but in everyday life.
References
Microfluidic Chip Development Services for Organ-On-A-Chip – Creative Biolabs
Introduction to Organs-on-a-Chip
A guide to the organ-on-a-chip | Nature Reviews Methods Primers

This is a good blog. It nicely demonstrates a good understanding of organ-on-a-chip technology and clearly explains its purpose and…
This is a good blog, very engaging with a good backgroud to 3D bioprinting. You could improve your blog with…
This is a good, very interesting blog about necrobotics. It explores the idea of necrobiotics which is fairly new approach…
This is a good blog. You introduce the reader to the topic of prosthetics and bionic limbs in a very…
This is a good blog introducing hernia mesh benefits and drawbacks. You create a narrative in this blog, which showcase…