Neurodegenerative disease has interested me for a while. Having had family with dementia and multiple sclerosis, I’ve known about them from a young age. After Nick Evan’s tissue engineering lecture, I thought about using tissue engineering to treat neurodegenerative diseases. Could we use biomaterials, 3D printing, or stem cells to regenerate lost or damaged neurones?
What are the possibilities?
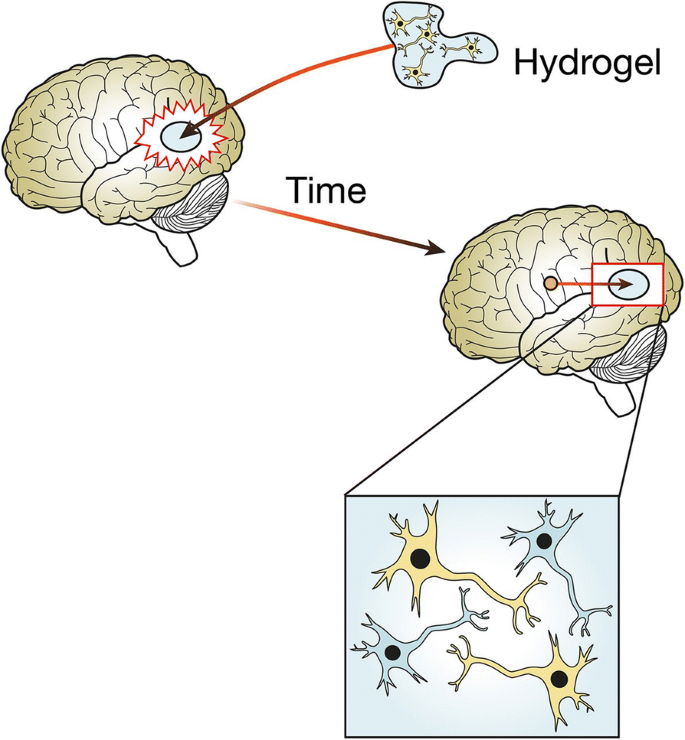
I began to look into how tissue engineering could be useful in treatment of these diseases, and found many approaches. For example, 3D printing bio-scaffolds- structures that allow cell growth and mimic the extracellular matrix. Essentially, a 3D structure which allows neurones to grow in the brain. The scaffold needs to be able to maintain integrity during implantation, not be toxic, allow neuronal migration and proliferation, allow electrochemical communication, and release substances in a controlled manner. One class of materials that looks promising is smart hydrogels. Due to their high water content, they mimic the soft tissue environment and respond to external stimuli. The scaffolds can release growth factors and provide stem cells for nerve regeneration. However, research is still in early stages, as so far, only collagen has been useful for nerve regeneration and hasn’t been implanted in patients. If research advanced, patients with neurodegenerative diseases could have this scaffold implanted to regenerate lost neurones (1).
One unique issue is that the adult brain is designed against neuronal regeneration. Neurones don’t regenerate once they’re lost and there are cell-secreted molecules which make sure of this. Any attempts to regrow neuronal tissue will face this problem and will need to avoid attack from microglia, the brain’s immune cells (2). If you want to introduce foreign material to a brain, you need a strategic and delicate approach.
Mini-brains to use as models?

Another interesting idea I found is that we could create mini-brains from stem cells and engineer them into neurones. This organoid could be used to model diseases like Alzheimer’s and test different treatments on it (3). Alzheimer’s disease is characterised by the build-up of amyloid plaques and tau in the brain (4). With mini-brains, mechanisms can be studied, and drugs can be tested to see if they reduce amyloid and tau build-up. Mini-brains could not only help us understand the disease, but could also be revolutionary for personalised medicine. If we use stem cells from individual patients, a personalised mini-brain could be created, allowing better treatment. This is such an exciting and innovative approach which highlights the possibilities of neural tissue engineering, and if successful in Alzheimer’s treatment, it can be used for other diseases.
What about the ethics?
It is also important to consider ethics in this research. If we get closer to engineering a lab-grown brain, could it have consciousness and feel pain and emotion? Also, current research aims to treat disease and injury, but could it be used to enhance cognitive abilities? Research is still far from these realities, but considering and putting measures in place ahead of time might not be in vain. My research into this topic exposed me to interesting ideas and I will follow the research to keep updated on how neural tissue engineering advances, and how ethics plays into the research being done.
Sources
- https://pmc.ncbi.nlm.nih.gov/articles/PMC10302050/
- https://royalsocietypublishing.org/doi/10.1098/rsif.2019.0505
- https://www.thenila.com/blog/innovative-mini-brains-could-revolutionize-alzheimers-treatment
- https://www.alzheimers.org.uk/about-dementia/types-dementia/alzheimers-disease
- https://jbiomedsci.biomedcentral.com/articles/10.1186/s12929-018-0491-8
- https://health.economictimes.indiatimes.com/news/industry/washington-3d-mini-brains-developed-in-lab/50995518

This is a good blog. It nicely demonstrates a good understanding of organ-on-a-chip technology and clearly explains its purpose and…
This is a good blog, very engaging with a good backgroud to 3D bioprinting. You could improve your blog with…
This is a good, very interesting blog about necrobotics. It explores the idea of necrobiotics which is fairly new approach…
This is a good blog. You introduce the reader to the topic of prosthetics and bionic limbs in a very…
This is a good blog introducing hernia mesh benefits and drawbacks. You create a narrative in this blog, which showcase…