My first real understanding of dementia came from an unexpected source – the movie The Notebook. As a teen, I was struck by how Alzheimer’s slowly erased Allie’s memories of Noah and their life together. It wasn’t just the romance that affected me, but the terrifying idea of losing one’s sense of self. That curiosity stayed with me, evolving from an emotional reaction into a scientific interest during my biomedical studies. What I once saw as an inevitable part of aging, I now recognise as a complex medical challenge – and one where emerging stem cell research might offer real solutions.
Understanding Dementia: A Global Challenge
Dementia is an umbrella term for conditions that cause cognitive decline, the most common being Alzheimer’s disease. It affects over 55 million people worldwide, according to the World Health Organization. The disease is characterised by the progressive destruction of neurons, leading to memory loss, impaired judgement, and personality changes. Despite extensive research, current treatments only manage symptoms rather than stopping or reversing the disease. Medications like cholinesterase inhibitors can temporarily slow progression, but no cure exists. This makes the search for innovative treatments, such as stem cell therapy, even more critical.
What Are Stem Cells?
Stem cells are unique cells with the remarkable ability to develop into different types of specialised cells in the body. They serve as the body’s natural repair system, capable of dividing and renewing themselves to replace damaged or lost cells. There are different types of stem cells, including embryonic stem cells, which can become any cell type, and adult stem cells, which have more limited differentiation potential. In recent years, induced pluripotent stem cells (iPSCs), adult cells that have been genetically reprogrammed to act like embryonic stem cells, have gained attention for their potential in regenerative medicine. Scientists hope to harness these cells to restore lost neurons and repair brain tissue in dementia patients.
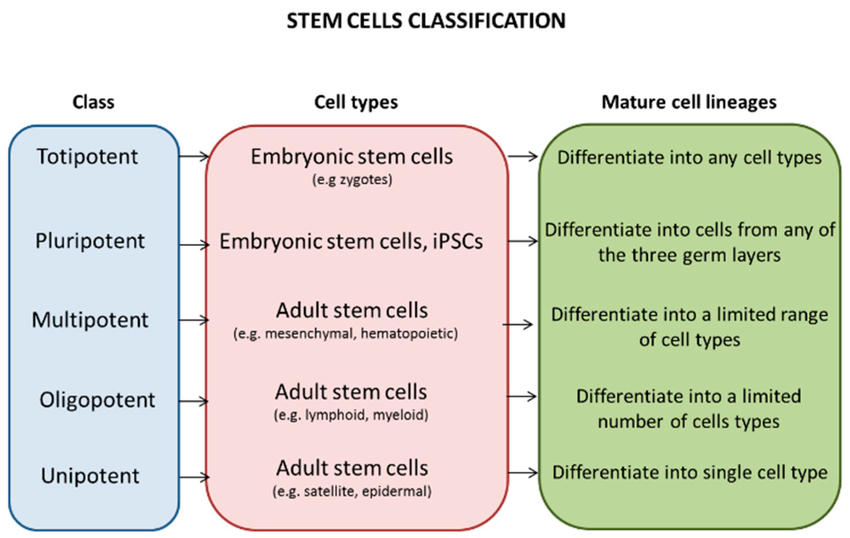
How Stem Cells Could Offer a Solution
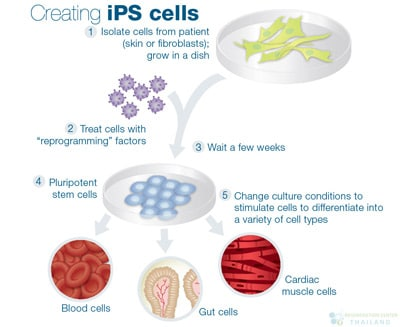
Stem cells offer an exciting avenue for dementia treatment because of their ability to develop into different types of cells, including neurons. A groundbreaking 2021 study in Nature Neuroscience demonstrated that transplanting iPSC-derived neurons into Alzheimer’s model mice not only restored memory but also reduced toxic amyloid plaques – hinting at a future where stem cells could both repair and protect the brain. Amyloid-beta plaques are sticky protein clumps that accumulate in the brains of Alzheimer’s patients, disrupting neuron function and triggering memory loss which makes them a key target for treatments. Researchers are also investigating mesenchymal stem cells (MSCs) for their anti-inflammatory and neuroprotective properties, which could slow disease progression. Early-stage clinical trials are assessing the safety and efficacy of these therapies in humans, with some patients showing improved cognitive function and brain regeneration. While still in experimental stages, this research suggests a future where we might be able to restore lost memories rather than just slow their decline.
A Hopeful Yet Cautious Outlook
Yet I remain cautiously optimistic. While stem cells offer groundbreaking potential to combat dementia, significant challenges remain – from ethical dilemmas to safety risks and the immense complexity of repairing the brain. We are far from a cure, but every medical breakthrough once seemed impossible. This research represents something The Notebook’s Allie never had: real hope.
Because in the end, this research isn’t just about preserving memories – it’s about preserving the love stories they hold. And that’s a battle Noah would understand.
Charities like Dementia UK and Alzheimer’s Research UK are racing to turn this science into cures, but they need help. If this blog touched you, please give today:
Sources
- Dementia treatment l Acetylcholinesterase inhibitors | Donepezil, Galantamine, Rivastigmine https://www.youtube.com/watch?v=yD4W-iAHfUo&ab_channel=Dr.PaulienMoyaert (accessed 24/03/25)
- Classification of stem cells https://www.researchgate.net/publication/333053144/figure/fig1/AS:11431281213162280@1702959742961/Classification-of-stem-cells-Stem-cells-can-be-classified-according-to-their-plasticity.tif (accessed 24/03/25)
- Creating induced pluripotent cells https://stemcellthailand.org/wp-content/uploads/2014/05/ips-pluripotent-cells.jpg (accessed 24/03/25)
This is a good blog. It nicely demonstrates a good understanding of organ-on-a-chip technology and clearly explains its purpose and…
This is a good blog, very engaging with a good backgroud to 3D bioprinting. You could improve your blog with…
This is a good, very interesting blog about necrobotics. It explores the idea of necrobiotics which is fairly new approach…
This is a good blog. You introduce the reader to the topic of prosthetics and bionic limbs in a very…
This is a good blog introducing hernia mesh benefits and drawbacks. You create a narrative in this blog, which showcase…