Imagine having the ability to “pick and mix” genetic traits – eye color, disease resistance, height maybe even intelligence. CRISPR gene editing means this isn’t just science fiction; it’s becoming science reality! Acting like a molecular toolkit to produce ‘designer babies’ offering the potential to select or modify traits before birth. If we can customize human life, who gets access?
What Got Me Interested in CRISPR
I became interested in CRISPR after reading about legal battles between major research institutions and ethical tensions between innovation and accessibility. Critically, this topic shows the intersection of cutting-edge technology and human rights. During research I was struck by the rapid progression from laboratory research to clinical trials and how tightly the therapeutic potential is limited through patents. It raised difficult ethical questions and concerns: should these technologies be monopolized or are they a global good?
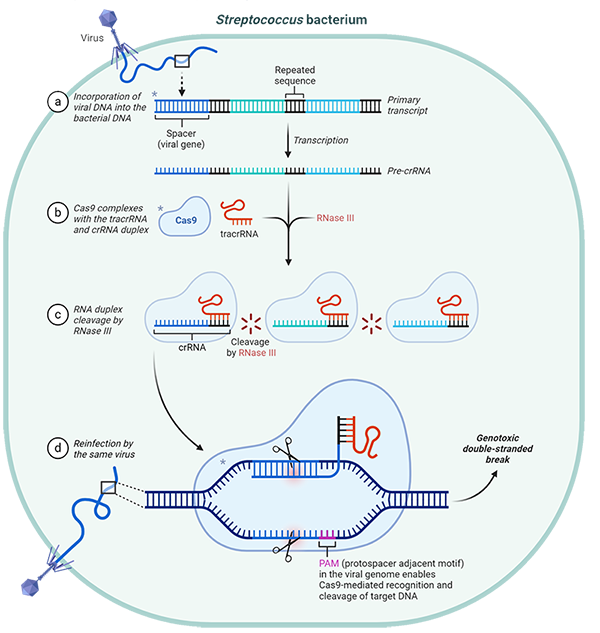
Figure 1. Image showing the mechanism of the CRISPR-cas9 enzyme (BioRad, 2021)
What is CRISPR-Cas9?
CRISPR-Cas9 is a genome editing system derived from the bacterial immune response. It functions as a precise, RNA guided tool for editing genes in living organisms (Doudna & Charpentier, 2014). Cas9 facilitates highly specific and precise genomic modifications by creating double-stranded breaks at targeted DNA sequences, directed by a strand of guide RNA. These breaks are repaired through homology-directed repair (HDR) or non-homologous end joining (NHEJ), these introduce small mutations (Barrangou & Doudna, 2016).
Who owns CRISPR?
One of the most intriguing controversies surrounding CRISPR isn’t scientific—it’s legal! Both the University of California and the Broad Institute contested ownership of the technology. Ultimately, the Broad Institute was awarded key patents. Critical examination shows a troubling dynamic; these exclusive patents restrict access and increase costs, limiting freedom of research (Correa & Hilty, 2022).
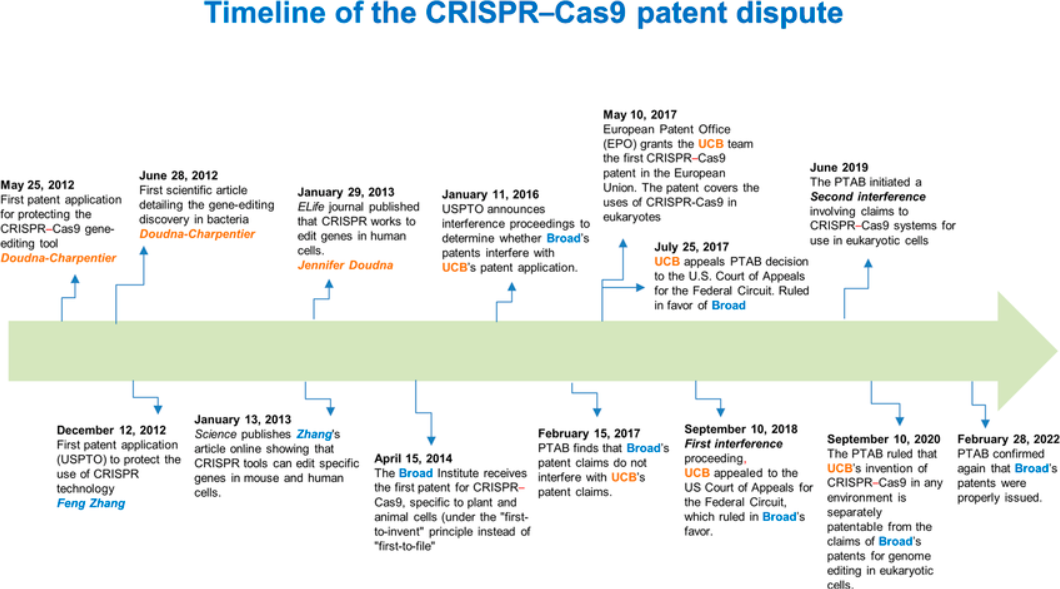
Figure 2, Timeline of the CRISPR CAS-9 patent dispute (Aquino-Jarquin, 2022)
Continued research led me to question whether patents hinder CRISPRs medical potential. Patents are widely regarded as incentives for innovation; however, they often prevent widespread accessibility to lifesaving treatments (Mir et al., 2022). Consequently, medical technology ownership through patents prioritizes profit over patient access. I considered open innovation and open-source models as ways to reduce patent exclusivity (Mali, 2020). This led me to pose the question: Should medical treatment be owned by a few or shared for the common good?
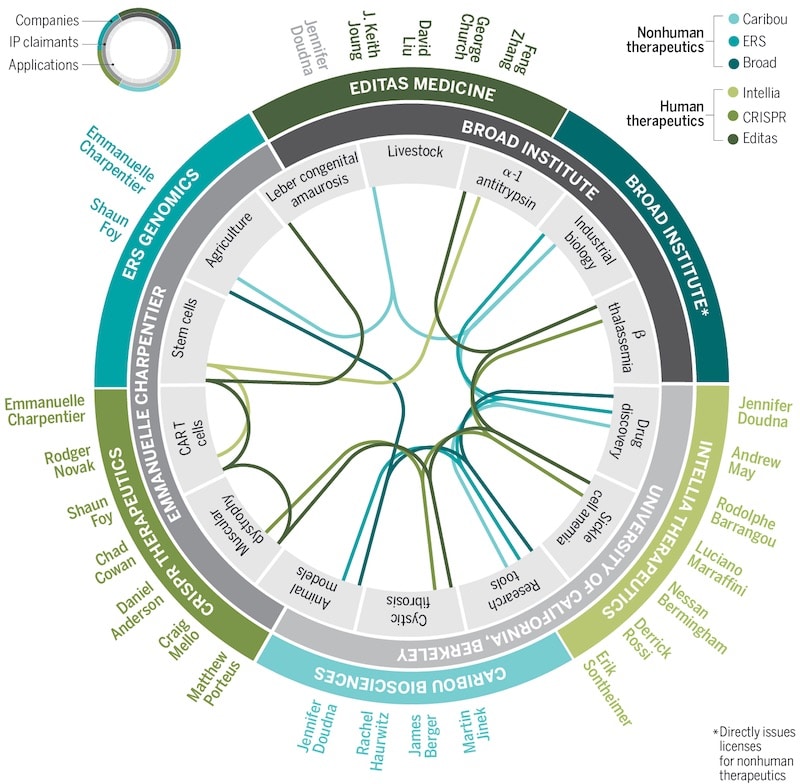
Figure 3. Shows the complex network of CRISPR-Cas9 intellectual property ownership, applications and licensing (Rodríguez Fernández, 2018).
Is it Ethical?
Gene editing offers remarkable possibilities but also raises challenging and uncomfortable questions. Core issues consent, accessibility and the potential societal impact. Genetic editing poses a risk for off-target mutations which are likely irreversible and raise significant concerns about human health and ecological stability in the long term (Evans, 2021).
Personally, researching these risks altered my perspective. Despite the exciting potential, I became concerned about unintended consequences. Irreversible changes impact entire ecosystems or human health across generations. I also confronted issues such as autonomy and distributive justice.
The National Academies in the US have approved germline editing for serious diseases in situations where it is safe and informed by parental consent. However, this may push technology closer towards practices such as eugenic enhancement. Germline editing brings forwards the issue of consent. Prompting me to question whether it is ethically justifiable to make permanent genetic decisions for individuals who are unable to give their consent (Waters, 2000).
The future of CRISPR
Researching this topic altered me to the balance between innovation and ethical responsibility. I concluded that achieving the full potential medical benefit of requires global collaboration and transparent regulatory frameworks. CRISPR could easily become a privilege reserved only for the affluent. Initiatives like patent pools can promote broader and affordable access, important for low and middle income countries. Flexible licensing models such as non-exclusive and humanitarian use licenses. Ultimately, I do not believe that CRISPRs success will be solely scientific but in the ethical, responsible and equitable deployment of this technology.
Bibliography
Aquino-Jarquin, Guillermo. ‘Early “Reduction to Practice” of the CRISPR–Cas9 Invention in Eukaryotic Cells’. Frontiers in Genetics 13 (4 October 2022). https://doi.org/10.3389/fgene.2022.1009688.
Barrangou, Rodolphe, and Jennifer A. Doudna. ‘Applications of CRISPR Technologies in Research and Beyond’. Nature Biotechnology 34, no. 9 (September 2016): 933–41. https://doi.org/10.1038/nbt.3659Bio-Rad. ‘How CRISPR Revolutionized Science’. Accessed 25 March 2025. https://www.bio-rad-antibodies.com/blog/how-crispr-revolutionized-science.html.
Correa, Carlos M., and Reto M. Hilty, eds. Access to Medicines and Vaccines: Implementing Flexibilities Under Intellectual Property Law. Cham: Springer International Publishing, 2022. https://doi.org/10.1007/978-3-030-83114-1.
Doudna, J. A., & CharpThe new frontier of genome engineering with CRISPR-Cas9. Science, 346(6213), 1258096. Centier, E. (2014).Evans, John H. ‘Setting Ethical Limits on Human Gene Editing after the Fall of the Somatic/Germline Barrier’. Proceedings of the National Academy of Sciences 118, no. 22 (June 2021): e2004837117. https://doi.org/10.1073/pnas.2004837117.
Mali, Franc. ‘Is the Patent System the Way Forward with the CRISPR-Cas 9 Technology?’ Science & Technology Studies 33, no. 4 (15 December 2020): 2–23. https://doi.org/10.23987/sts.70114.
Mir, Tahir Ul Gani, Atif Khurshid Wani, Nahid Akhtar, and Saurabh Shukla. ‘CRISPR/Cas9: Regulations and Challenges for Law Enforcement to Combat Its Dual-Use’. Forensic Science International 334 (May 2022): 111274. https://doi.org/10.1016/j.forsciint.2022.111274.
Bio-Rad. ‘How CRISPR Revolutionized Science’. Accessed 25 March 2025. https://www.bio-rad-antibodies.com/blog/how-crispr-revolutionized-science.html.
This is a good blog. It nicely demonstrates a good understanding of organ-on-a-chip technology and clearly explains its purpose and…
This is a good blog, very engaging with a good backgroud to 3D bioprinting. You could improve your blog with…
This is a good, very interesting blog about necrobotics. It explores the idea of necrobiotics which is fairly new approach…
This is a good blog. You introduce the reader to the topic of prosthetics and bionic limbs in a very…
This is a good blog introducing hernia mesh benefits and drawbacks. You create a narrative in this blog, which showcase…