
Parkinson’s disease is a neurogenerative disorder caused by the loss of nerve cells in a part of the brain called substantia nigra. Losing these cells causes a drop in the abundance of a vital chemical called dopamine, leading to a whole host of symptoms, including tremors, stiffness, and dementia. Parkinson’s disease can also strongly impact the mental health of affected people, often associated with depression and anxiety.
This disease is common, affecting more than 1 in 50 people over the age of 65, with some people even experiencing symptoms under 40 years old. The cause of the degeneration of specific nerve cells associated with Parkinson’s disease is unknown, but there is evidence for some new approaches for its treatment using stem cells.
How could stem cells be used?
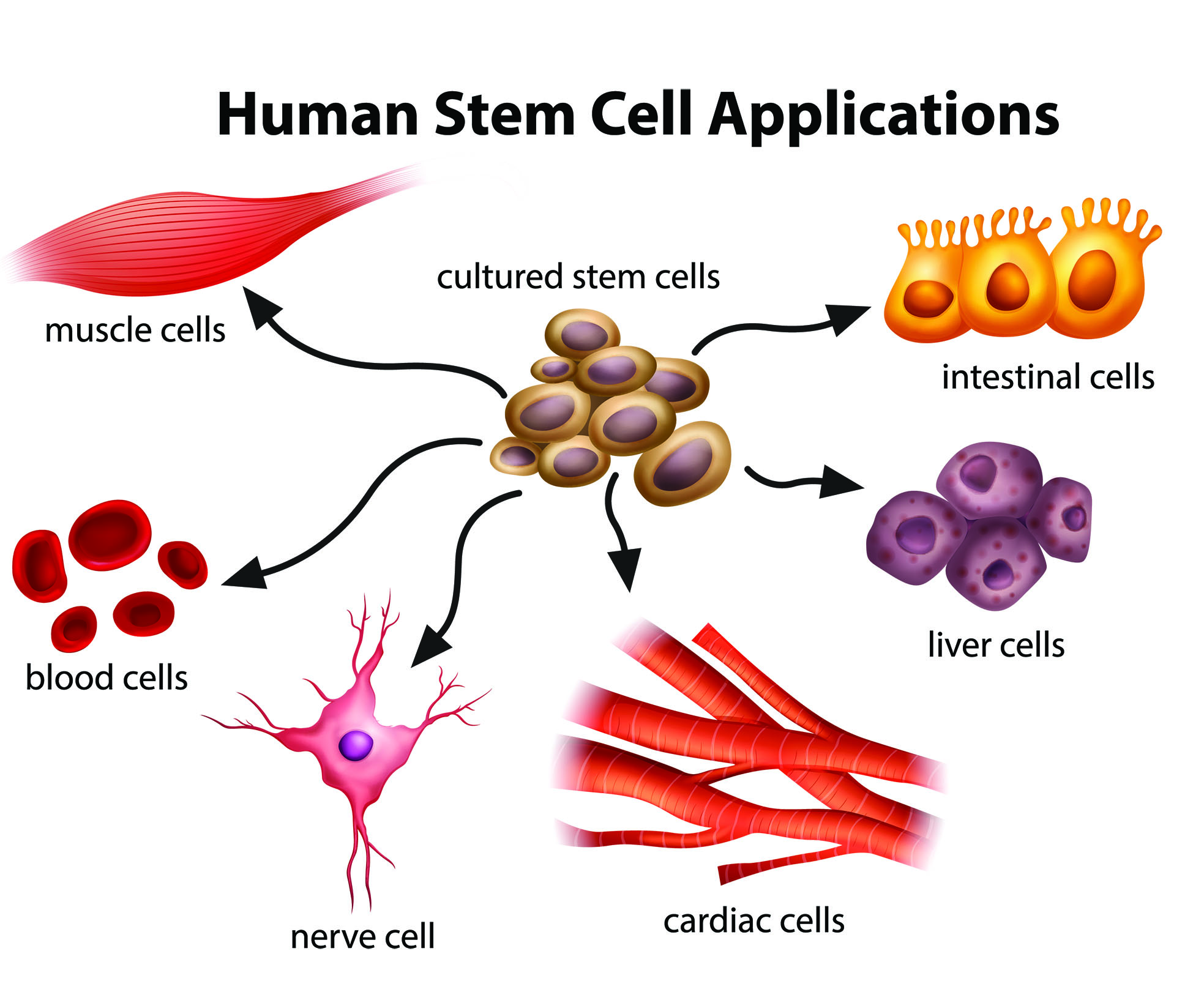
First, what are stem cells? There are a few kinds of stem cell, but the term generally refers to cells that are capable of developing into other types of cell. Embryonic stem cells, in particular, are pluripotent, meaning they can give rise to all cell types. Induced pluripotent stem cells (iPSCs), which also differentiate into any cell type, can be created via the reprogramming of existing adult cells. Of all stem cells available, these two seem to be the most promising.
One major therapy which has the potential to treat Parkinson’s disease is “Bemdaneprocel”, or “BRT-DA01”. In this therapy, human embryonic stem cells are converted into dopamine-producing neuron progenitors in the lab before being surgically implanted into a specific area of the brain. These cells may be recognised as foreign, so patients must take immunosuppressants for a year. This method began a Phase 1 trial in 2021. “BRT-DA01” showed high safety and potential clinical benefits, and is expected to undergo a Phase 3 trial in the first half of this year.
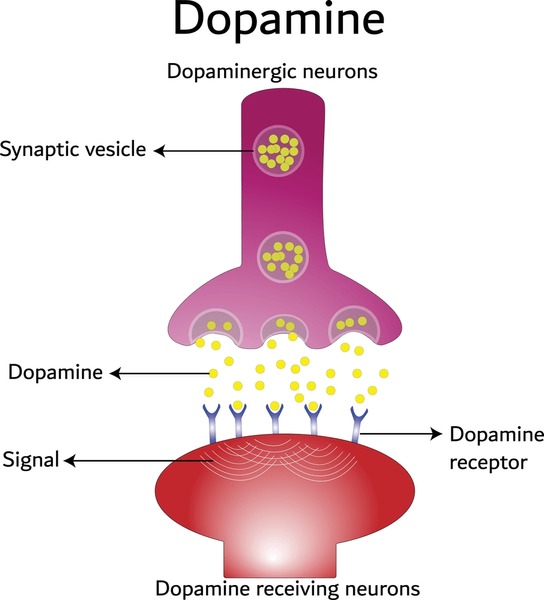
Another experimental therapy for Parkinson’s disease which aims to replace lost dopaminergic neurons was approved for a Phase 1/2a trial in the US by the FDA in late 2023. In this method (“ANPD001”), skin cells are collected from a patient and reprogrammed in the lab, producing iPSCs which are then converted into immature dopamine neuronal precursor cells. Finally, they are transplanted into the brain, in the hope that they will develop into those crucial neurons. One key advantage of “ANPD001” over “BRT-DA01” is that iPSCs are derived from the patient, so immunosuppressants aren’t needed. As of January this year, this method has been safely carried out with two patient cohorts.
Some ethical issues

While embryonic stem cells may be an effective source for the treatment of Parkinson’s, testing and using them for science raises some interesting ethical questions. These cells are derived from early-stage embryos, in a process involving the destruction of the embryo. The moral status of the embryo in comparison to human life therefore must be questioned. Furthermore, embryos used for scientific purposes are often donated by people undergoing in vitro fertilisation. I feel it is crucial that donors are entirely aware of how their embryos will be used, and clear consent is given.
This is obviously a difficult ethical dilemma, though there seems to be a work-around. Fortunately, iPSCs avoid the ethical questions faced by embryonic stem cells, because they are not derived from embryos. As a result, these cells seem to be a far more acceptable choice for the treatment of Parkinson’s disease, especially from the standpoint of public concern.
This is a very well-researched and informative blog with good structure and use of images. It could be improved by being a bit more reflective. What did you learn while researching this topic? What are your thoughts and opinions on the ethical considerations and the promise of this new technology?