Aging is an inevitable part of life, but what if we could slow it down – or even reverse it? Scientists are exploring the potential of stem cells to unlock the secrets of aging, offering the exciting possibility of longer and healthier lives. Through lectures and discussions, I’ve come to appreciate how stem cell research pushes the boundaries of longevity. But how realistic is this, and what role do stem cells play in the pursuit of eternal youth?
Why Do We Age?
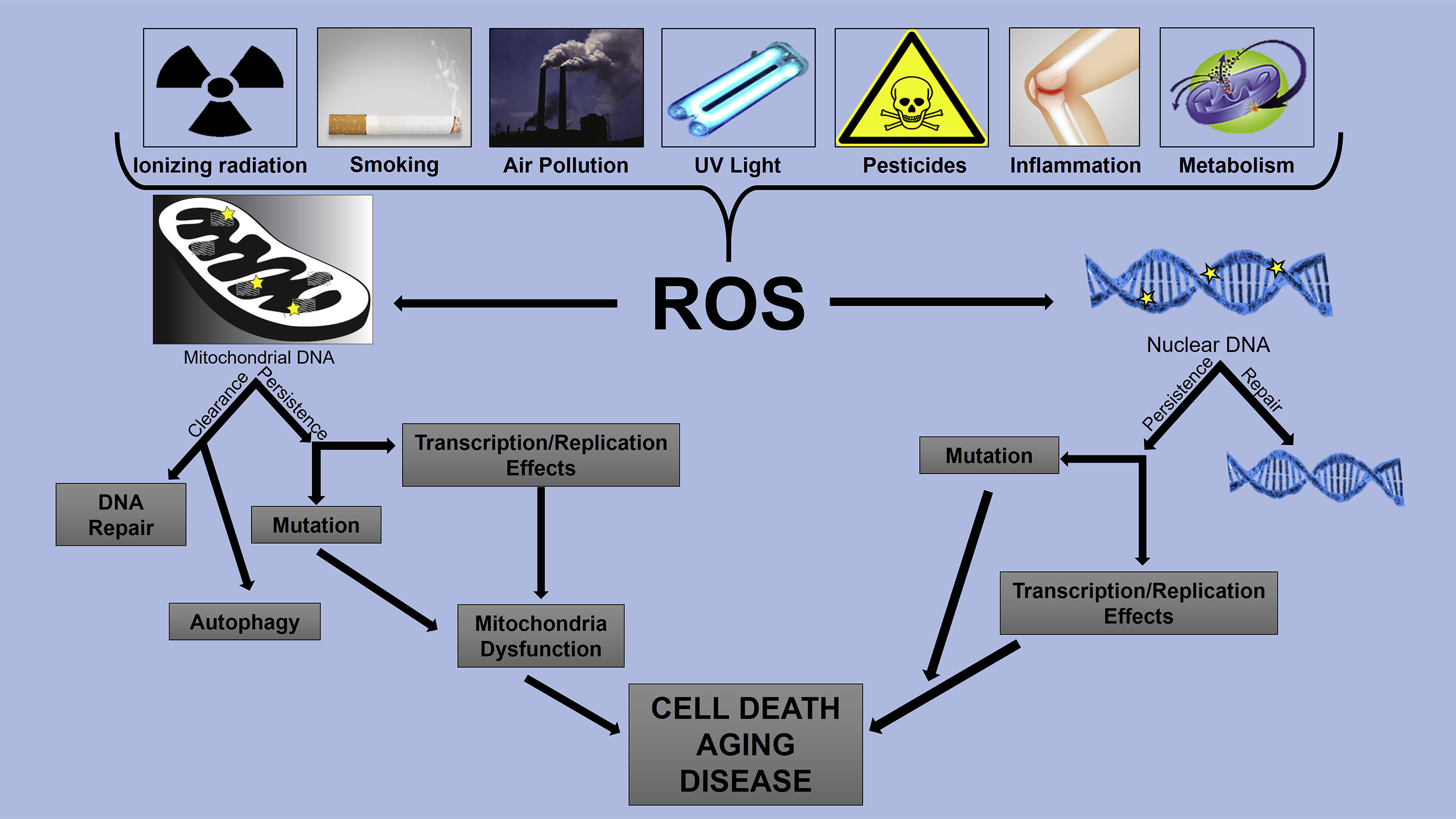
Aging happens as our cells accumulate damage over time. DNA mutations, oxidative stress (related to too many reactive oxygen species), and the shortening of telomeres (the protective caps on our chromosomes) all contribute to tissue decline. Stem cells, which can develop into different cell types, naturally diminish with age, reducing the body’s ability to repair itself.
Stem Cells as a Fountain of Youth
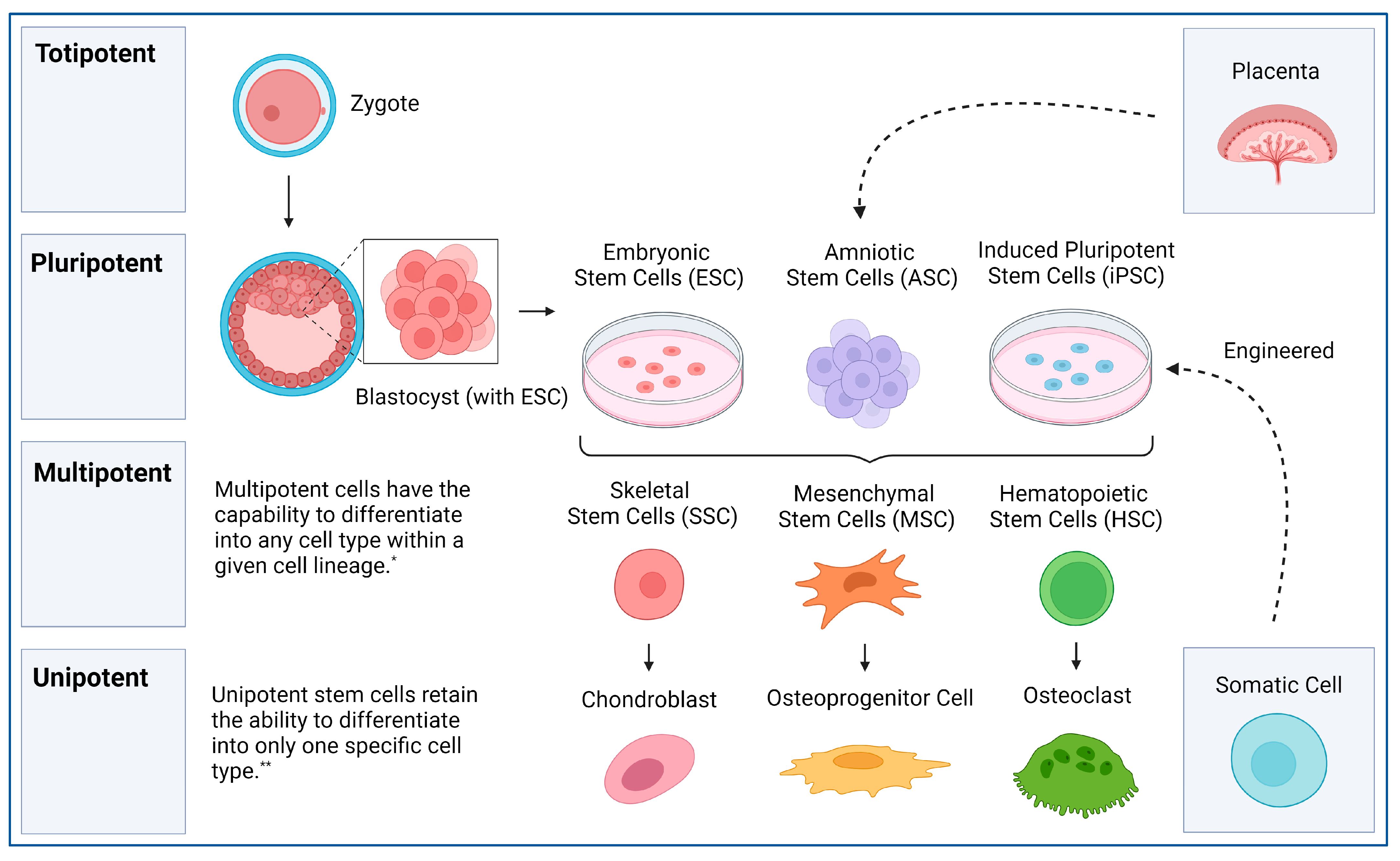
Scientists are investigating whether replenishing or rejuvenating stem cells could combat aging. Mesenchymal stem cells (MSCs), found in bone marrow and fat tissue, secrete growth factors that aid tissue repair. Studies suggest they could improve skin elasticity, reduce wrinkles, and even regenerate damaged organs. MSCs are already in clinical trials for treating age-related frailty and inflammation.
Another promising option is induced pluripotent stem cells (iPSCs), adult cells reprogrammed into a stem-like state. In mouse studies, researchers extended lifespan and reversed signs of aging by introducing rejuvenated cells. iPSCs could replace aged or damaged cells, rejuvenating tissues without the need for donors. In discussions, we debated whether pursuing cellular youth might create societal imbalances, benefiting the wealthy while leaving others behind.
Stem Cell Therapies for Aging-Related Diseases
Stem cells might not just help with wrinkles – they could tackle diseases of aging. For instance, stem cell transplants are being explored for neurodegenerative conditions like Alzheimer’s and Parkinson’s. By generating healthy neurons, scientists hope to replace the lost brain cells causing these diseases.
Stem cells also show promise for cardiovascular disease. Researchers are working on generating new heart muscle cells from iPSCs, which could be transplanted into damaged hearts. Similarly, MSCs are being tested to repair cartilage in osteoarthritis patients, offering hope for those with joint pain and reduced mobility.
Rejuvenating the Skin and Immune System
One of the most visible signs of aging is skin deterioration. Stem cell-based treatments, like exosome therapies (using stem cell-derived vesicles filled with growth factors), aim to boost collagen production, improve skin texture, and enhance overall skin resilience.
Stem cell therapies may also rejuvenate the immune system. The thymus, which produces immune cells, shrinks with age, weakening immunity. Researchers are exploring whether stem cell injections could regenerate thymic tissue, restoring immune function and boosting longevity.
The Ethical and Practical Challenges
Despite the promise, stem cell therapies pose ethical and logistical challenges. While iPSCs bypass the controversy of embryonic stem cells, safety remains a concern. Unchecked cell growth could cause cancer, and immune responses to transplanted cells must be addressed. The technology is costly and primarily accessible through clinical trials, raising questions about equitable access – a point that sparked intense class discussions.
From a societal perspective, extended lifespans prompt complex questions: are we prepared for a world where people live to 120 or beyond? How might this affect resources and healthcare systems? These conversations made me reflect on whether the goal should be radical life extension or enhancing health span so people age with dignity and vitality.
A Glimpse Into the Future
While we’re not close to immortality, stem cells offer a promising path to healthier aging. As research progresses, therapies could shift from experimental to routine, helping people live longer, more vibrant lives. The idea of eternal youth may not be science fiction forever – with stem cells, it just might become reality.
Would you want to know what you’d look like at 150? The future of aging is unfolding, and stem cells are at the heart of the revolution.
Sources
- DNA damage by oxidative stress: Measurement strategies for two genomes https://ars.els-cdn.com/content/image/1-s2.0-S2468202017301341-gr1_lrg.jpg (accessed: 06/03/2025)
- Stem Cells and Acellular Preparations in Bone Regeneration/Fracture Healing: Current Therapies and Future Directions https://www.mdpi.com/cells/cells-13-01045/article_deploy/html/images/cells-13-01045-g001.png (accessed 07/03/2025)
- What is the therapeutic potential of exosomes? https://www.youtube.com/watch?v=NQeY_oIMNII&ab_channel=ScienceAnimated (accessed 07/03/2025)




This is a good blog. It nicely demonstrates a good understanding of organ-on-a-chip technology and clearly explains its purpose and…
This is a good blog, very engaging with a good backgroud to 3D bioprinting. You could improve your blog with…
This is a good, very interesting blog about necrobotics. It explores the idea of necrobiotics which is fairly new approach…
This is a good blog. You introduce the reader to the topic of prosthetics and bionic limbs in a very…
This is a good blog introducing hernia mesh benefits and drawbacks. You create a narrative in this blog, which showcase…