Before the stem cells lecture, I was not aware of inter-species chimeras. During the lecture it was proposed that they could be the solution to organ shortages. I found this an interesting proposition, and imagined a world where no one dies waiting for an organ. However, the thought growing of organs in another animal made me uneasy, and since learning about it, I’ve been wondering whether I would accept one if I needed it.
So what are they?
An inter-species chimera is an organism containing cells from two or more genetically distinct species. After further research I found that pigs are the most promising candidates for this research due to similarities in organ size.
Process of their creation for organ transplantation:
Video: me
As I learned more, I gained an appreciation for the innovation of genetic engineering. In theory, inter-species chimeras could be used to provide unlimited transplantable organs, but at what cost?
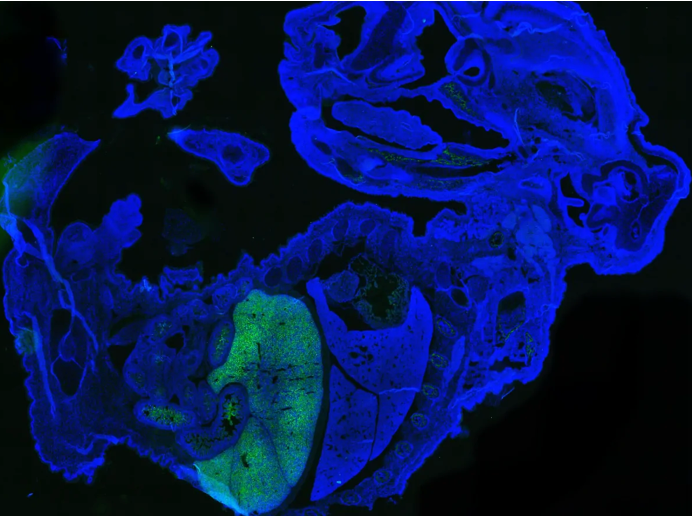
Image: mouse-human chimeric embryo with human cells labelled with green fluorescent protein by Jian Feng [1]
How far can we really go?
Despite promise with recent experiments forming tissues, inter-species chimeras face massive challenges. The high risk of organ rejection reminded me how fragile biology is and how even innovations struggle to overcome nature.
There are also numerous ethical concerns. How does this technology challenge our perspectives on humanity and morality? I thought about how others would react to the process of their creation. Recognising the controversy of this topic, I surveyed the public to gauge their opinions.
“The most egregious abuses of medical research” – George W. Bush describing human–animal chimeras [2]
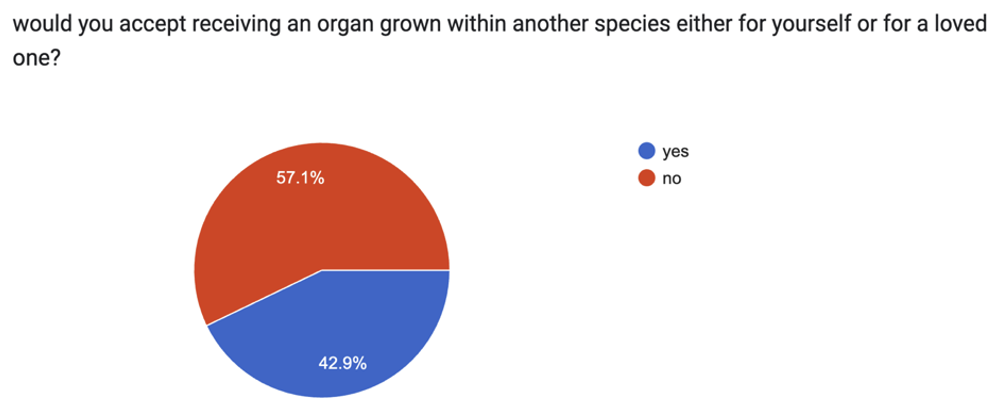
The results were evenly split, reflecting the diverse range of beliefs that should be considered.
Reasons ranged from fear of the unknown to ethical concerns. Many of those in favour argued saving lives justifies their use. Chimera creation involves genetic modification and experiments that could cause pain or distress to animals. Unlike human, animals cannot give consent. Is it ethical to use them for experiments solely for human benefit? Before their use, key concerns must be addressed first.
“The more you can show that it stands to produce something that will actually save lives … it shifts the scale of risk-benefit assessment in favour of pursuing research and away from those concerns that are more philosophical” – Vardit Ravitsky, a bioethicist [3]
To alleviate concerns, regulatory measures could be enhanced, implementing welfare assessments. Involving the public in decision-making would also help align advancements with societal values. However, even different countries have different opinions, and religious and cultural perspectives are also highly diverse.
It is clear that this topic needs to be approached sensitively, considering both individual beliefs and societal values.
What other choice do we have?
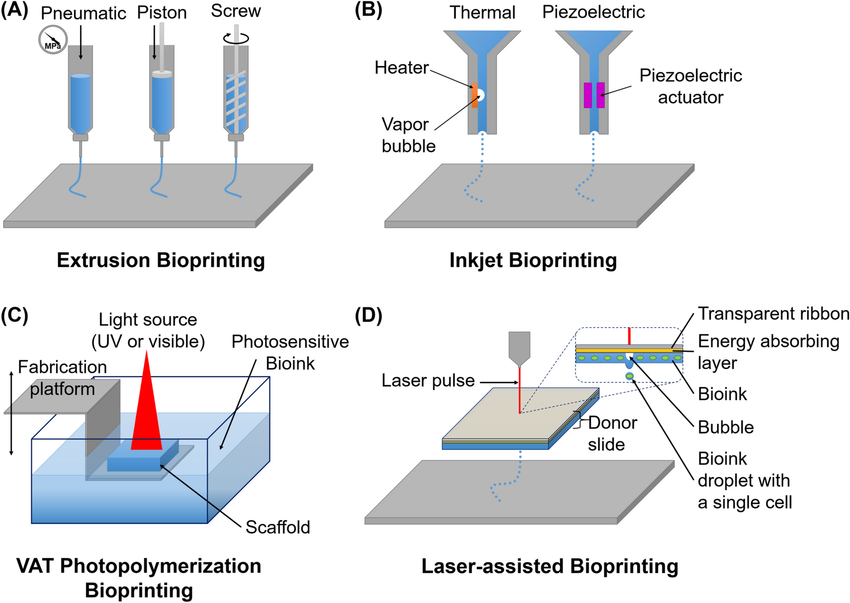
The demand for organ transplants is not decreasing. So, what are our other options? One possible solution is in-vitro stem cells allowing for patient-specific organs. Integrating techniques such as 3D bio-printing could also help us move towards a brighter future. [4]

Image: 3D bio-printing used to synthesise tissues [5]
My final thoughts
I’m torn between saving lives and animal welfare. After more research, the complexity of the issues is only more apparent. The number of articles expressing concerns has greatly impacted my perspective, highlighting the key considerations needed before implementation.
I believe we should innovate, but within boundaries. Should we set clear limits on inter-species organ growth, and if so, what should those be? Whilst some have argued the potential to save lives outweighs concerns, I don’t think it is that clear cut. The question isn’t just whether we can create inter-species chimeras, but whether we should.
References:
[1] https://www.the-scientist.com/chimera-research-opens-new-doors-to-understanding-and-treating-disease-71254
[2] https://www.pnas.org/doi/10.1073/pnas.1615787113
[3] https://www.washingtonpost.com/news/speaking-of-science/wp/2017/01/26/scientists-create-a-part-human-part-pig-embryo-raising-the-possibility-of-interspecies-organ-transplants/
[4] https://www.sir.advancedleadership.harvard.edu/articles/engineering-high-tech-solutions-organ-shortage#:~:text=Joseph%20Vacanti%2C%20Professor%20of%20Surgery,an%20interdisciplinary%20field%20that%20applies
[5]https://blogs.manchester.ac.uk/mioir/2023/06/09/can-3d-bioprinting-cure-organ-trafficking/





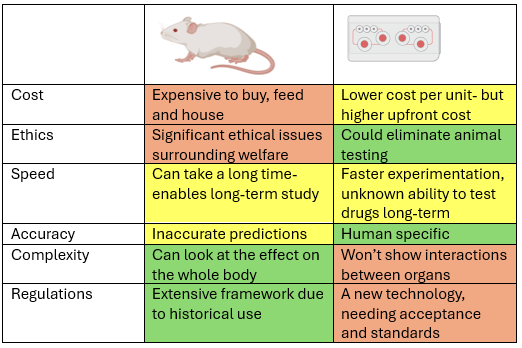
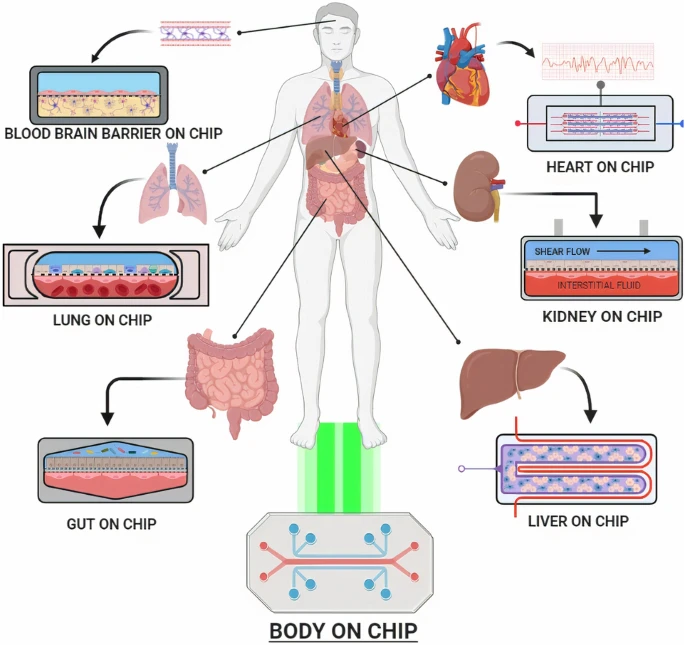




This is a good blog. It nicely demonstrates a good understanding of organ-on-a-chip technology and clearly explains its purpose and…
This is a good blog, very engaging with a good backgroud to 3D bioprinting. You could improve your blog with…
This is a good, very interesting blog about necrobotics. It explores the idea of necrobiotics which is fairly new approach…
This is a good blog. You introduce the reader to the topic of prosthetics and bionic limbs in a very…
This is a good blog introducing hernia mesh benefits and drawbacks. You create a narrative in this blog, which showcase…