The use of microbubbles within cancer treatment sounds comical, but these little bundles of joy can be used for a variety of medical applications. Over the last decade, these have been praised as the future of Cancer diagnosis and treatment and represent a safe and non-invasive alternative to Chemotherapy. I first heard about Microbubbles at a Workshop as part of my University, and it fascinated me that Microbubbles could kill cancer cells while sparing healthy ones, unlike Chemotherapy. This piqued my interest, which made me want to learn how they can be used to treat cancer within patients.
What are Microbubbles?
At the Workshop, I learned that Microbubbles are small bubbles (0.5–10 μm) consisting of a phospholipid outer layer and gas core and are used clinically during Ultrasound Imaging. When injected during Ultrasound Imaging, they resonate vigorously under the transducer, reflecting waves more effectively in body tissues and increasing imaging sensitivity.
![[9] An image showing the structure of a microbubble responding to ultrasound waves produced by a Transducer during ultrasound scanning in Pregnancy](https://generic.wordpress.soton.ac.uk/uosm2031-2025/wp-content/uploads/sites/529/2025/03/image-64.png)
However, once researchers realised these bubbles could move around the body safely, drug delivery in the treatment of cancer was greatly considered.
So Why Are These Tiny Bubbles So Effective In Treating Cancer?
Currently, chemotherapy drugs are injected into the blood and destroy cancer cells, however, this can also result in the death of healthy cells, leading to shocking side effects such as nausea and hair loss.
Microbubbles, on the other hand, can target smaller doses of chemotherapy drugs to cancer cells alone due to their Phospholipid shell, preventing the damage of healthy cells, reducing side effects, allowing patients to recover quicker.
“By using microbubbles and ultrasound we can control when and where a drug gets released, and crucially also distribute it throughout a tumour”
– Oxford scientist Eleanor Stride To WIRED
However, within aggressive types of cancer, low oxygen levels in tumours cause resistance to chemotherapy drugs, so to resolve this, Microbubbles can be filled with oxygen to improve the delivery of the drug and its ability to kill more aggressive cancer cells.
I was glad to read that the use of oxygen within microbubbles has also seen some effectiveness in reducing Cancer within rats, however, this is still within the pre-clinical research stage. Overall, there is some promise, but it was shocking to read that a separate stroke study revealed safety concerns when high dosages caused haemorrhaging in two patients, showing that parameters of ultrasound radiation and the number of microbubbles when applied should be evaluated to prevent this from ever happening again.
So Microbubbles May Have The Potential To Cure Cancer?
Despite this, many reports have reported positive results within the use of Microbubbles, giving it the potential to save many lives. At first, I found it comical that Microbubbles could be used to treat cancer, but my mind has seriously changed after reading different articles and papers within this field.
I found it fascinating when Oxford scientist Dr Stride told New Scientist that “If you expose the blood-brain barrier to bubbles and ultrasound, you can temporarily and reversibly enhance its permeability, which is potentially interesting for a lot of brain treatments”, which made me believe that Microbubbles deserves further research as it may also have the potential to protect brain cells from dying. I’m excited to witness what I’ve called the ‘bubble revolution’ taking shape within the NHS, and seeing the countless lives that will be transformed thanks to this ground-breaking research.
“Combining oxygen-carrying microbubbles with ultrasound-triggered delivery to solid tumours is a novel approach to enhancing tumour oxygenation and sensitivity to radiation, and it deserves further study,”
Dr. Bernhard Eric Bernhard, Ph.D., chief of the Radiotherapy Development Branch in NCI’s Division of Cancer Treatment and Diagnosis.
Bibliography
says CP. What are Microbubbles? [Internet]. News-Medical.net. 2018. Available from: https://www.news-medical.net/life-sciences/What-are-Microbubbles.aspx
New Portfolio. Editor’s choice: microbubbles [Internet]. Nature. 2022 [cited 2025 Mar 27]. Available from: https://www.nature.com/collections/edfggagdej
NCI Staff. Using Oxygen “Microbubbles” To Improve Radiation Therapy – National Cancer Institute [Internet]. www.cancer.gov. 2018. Available from: https://www.cancer.gov/news-events/cancer-currents-blog/2018/microbubbles-radiation-breast-cancer
Medeiros J. Using microbubbles to target cancer tumors [Internet]. WIRED. 2017 [cited 2025 Mar 27]. Available from: https://www.wired.com/story/cancer-bubble/
Hu Q, Zhang Y, Fu L, Xi Y, Ye L, Yang X, et al. Progress and preclinical application status of ultrasound microbubbles. Journal of Drug Delivery Science and Technology [Internet]. 2024 Feb [cited 2024 Oct 2];92:105312. Available from: https://www.sciencedirect.com/science/article/pii/S1773224723011644
Leeds Alumini. Microbubbles Animation [Internet]. Youtu.be. 2025 [cited 2025 Mar 27]. Available from: https://youtu.be/vXjeJQy6V_M?si=fJBXRXIMWAt58_sf
Spencer B. The tiny bubbles filled with drugs that could transform cancer treatment [Internet]. Mail Online. Daily Mail; 2015 [cited 2025 Mar 27]. Available from: https://www.dailymail.co.uk/sciencetech/article-3123944/The-tiny-bubbles-filled-drugs-transform-cancer-treatment-Findings-reduce-effects-chemotherapy.html
Macrae F. Bubbles “could deliver stroke drugs directly to the brain” [Internet]. Mail Online. Daily Mail; 2010 [cited 2025 Mar 27]. Available from: https://www.dailymail.co.uk/health/article-1328644/Bubbles-deliver-stroke-drugs-directly-brain.html
Rumney R. Workshop – Stem cell regenerative medicine – Robin Rumney [Internet]. Blackboard. 2025 [cited 2025 Mar 27]. Available from: https://blackboard.soton.ac.uk/ultra/courses/_228111_1/outline/edit/document/_7135192_1?courseId=_228111_1&view=content&state=view


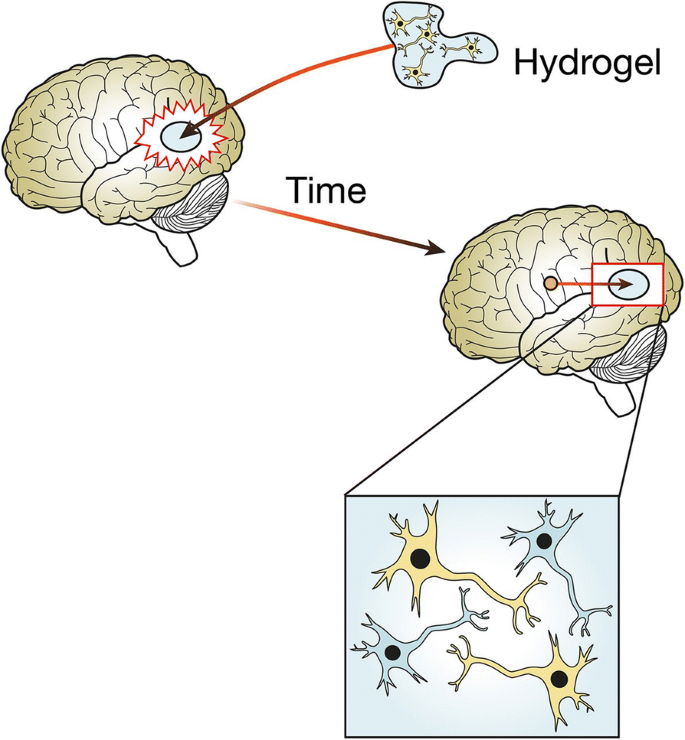

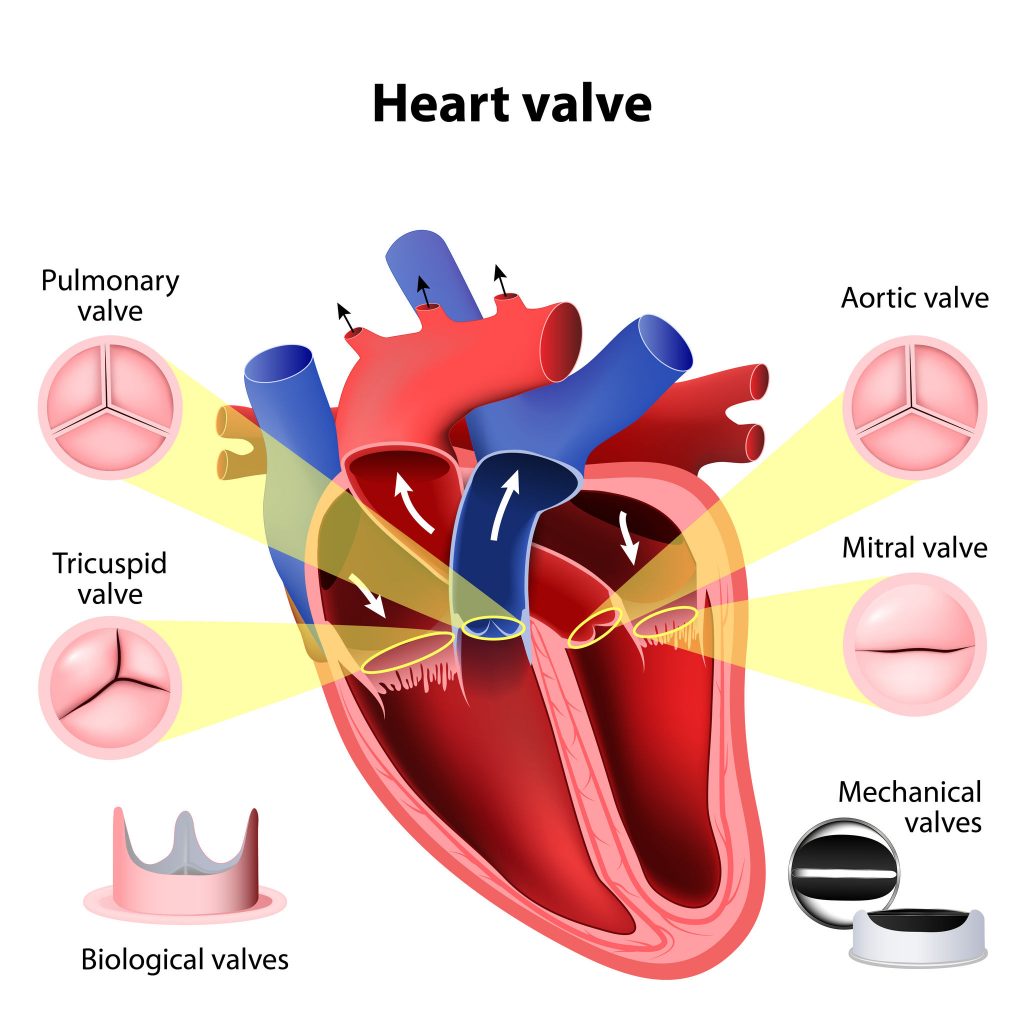
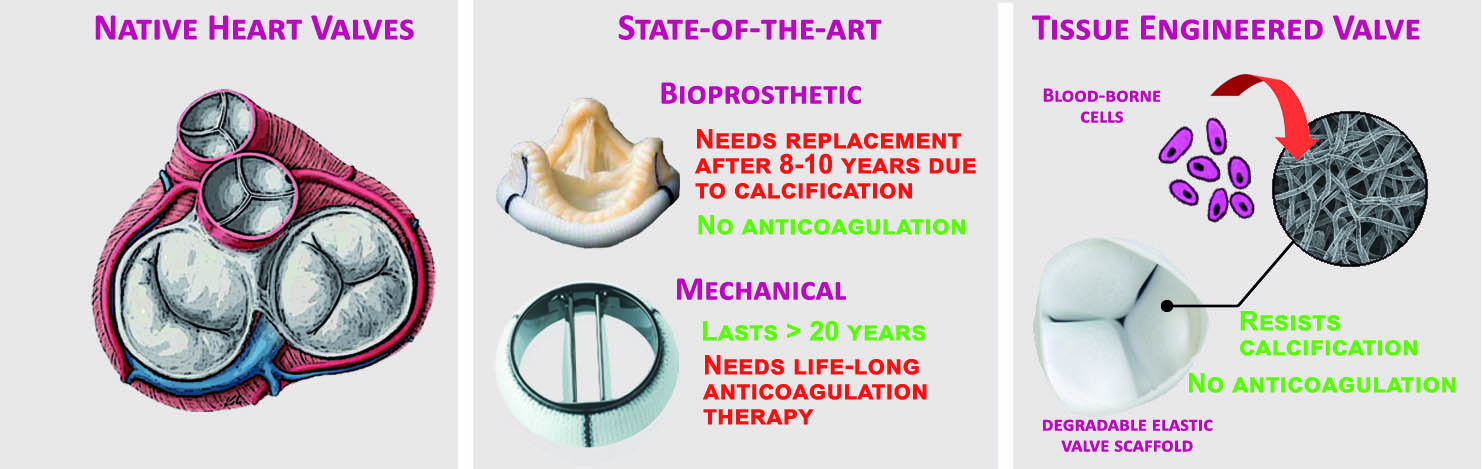





This is a good blog. It nicely demonstrates a good understanding of organ-on-a-chip technology and clearly explains its purpose and…
This is a good blog, very engaging with a good backgroud to 3D bioprinting. You could improve your blog with…
This is a good, very interesting blog about necrobotics. It explores the idea of necrobiotics which is fairly new approach…
This is a good blog. You introduce the reader to the topic of prosthetics and bionic limbs in a very…
This is a good blog introducing hernia mesh benefits and drawbacks. You create a narrative in this blog, which showcase…