Introduction:
While studying for a debate regarding the use of embryonic stem cells (ESCs) in research, I stumbled across articles about organoids. I remembered them being mentioned in one of our earlier lectures, therefore these articles peaked my interest. So I dug deeper. What interested me was the slightly morbid idea of growing a human brain and what that means. This digging led to another branch of this scientific, dystopian tree: brain chimeras. In this blog, I will be discussing why brain organoids and chimeras are made, what they are used for and what ethical issues rise.
Are we as scientists, justifying the use of these chimeras the same way Dr. Frankenstein justified the creation of his monster, selfishly in pursuit of greater knowledge despite the pain and suffering this may create?
What are they and how are they made?
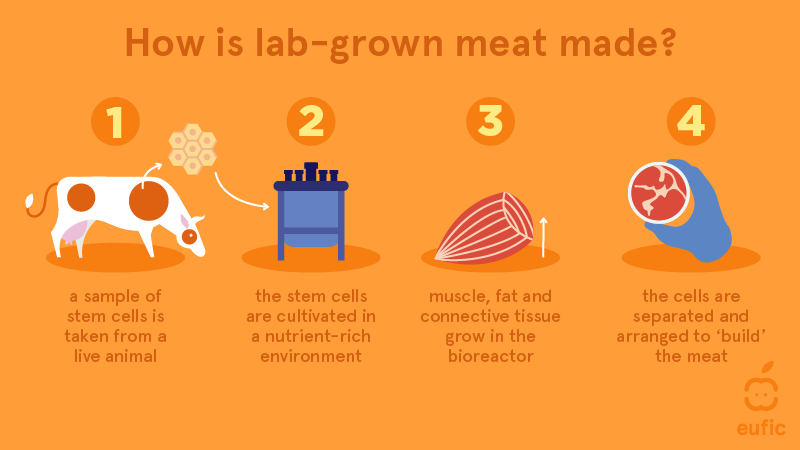
Organoids are 3D, self-organised tissue cultures made from stem cells. They are made by using iPSCs (induced pluripotent stem cells) which are made from adult somatic cells. This is done by reprogramming these somatic cells using transcription factors to make the cell pluripotent similar to ESCs.
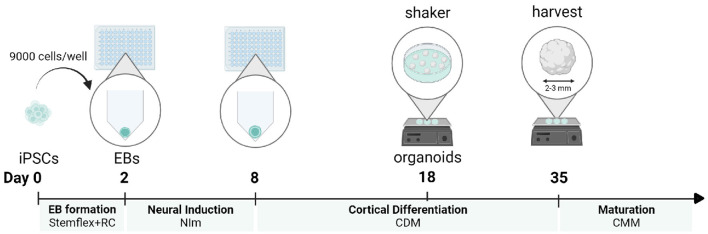
To grow these organoids the iPSCs are cultured to form an embryoid body (EB) which mimics the features of an early stage embryo. This is exposed to different mediums to encourage differentiation into brain cells then grown in a growth medium.
Organoids can be made with human stem cells and animal stem cells. This results in brain Chimeras which are made by engrafting human foetal tissue/iPSCs into a neonatal animal (e.g. mouse) brain.
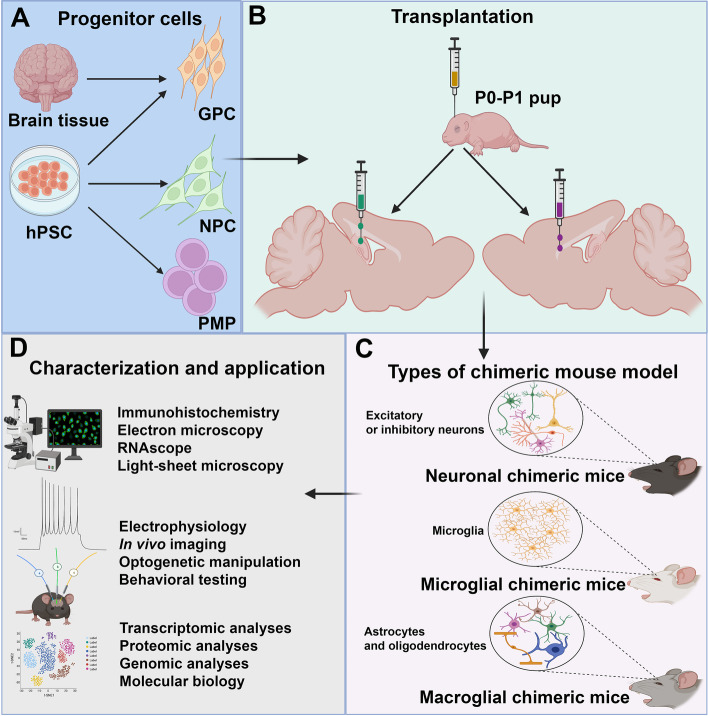
Why are they important?
We all know someone who is battling with a neurological condition. Many of these conditions are debilitating, ruin lives and have high mortality rates. This highlights the significance of this research.
One in two of us will suffer with dementia in our lifetime. Therefore, research into neurodegenerative disease is more important than ever before.
Although these organoids and human-animal brain chimeras are relatively new there has been a tremendous volume of studies which use this technology. They can offer important insight into the progression of neurodegenerative disease leading to new treatments. However, one could argue that these ‘brains’ are still fundamentally different and less complex than a real human brain as most animals are biologically very different and display different symptoms of neurological problems.
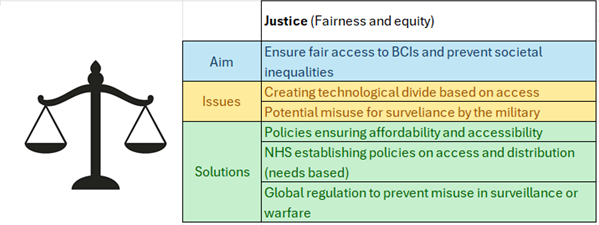
The issue
The main debate lies with the idea that human brain organoids or human-animal brain chimeras could be capable of intelligent thought. This raises many concerns as currently organoids hold no legal or moral rights within science. Are these chimeras thinking the same as a human may think? Are they self aware? More complex than say an ordinary mouse? And why should this matter?
There are significant regulations when using hESCs for research, such as the 14 day rule, however, organoids and human-animal chimeras are not regulated the same way, it does not even require a license as long as it does not use hESCs. Why is this?
These brain organoids and chimeras have the same moral status as many animals that are used in research. Should this be the case?
There is no right answer here as you cannot possibly know what a chimera or brain organoid could be thinking.
Conclusions and Reflections
Prior to researching this topic I did not appreciate the complexity and possible future of these models, I had a similar initial reaction to using any animal model; that it is a cruel and disturbing idea. However, animal models and hESCs have contributed greatly to the world of science, without them many treatments (HIV medication, cancer medications, vaccines) would not have been developed. This shows how important in vivo research is and these organoid/chimeric models may just add to the possibilities.
Contrary to this, I was surprised to find that making an organoid does not require any sort of formal license. I believe organoids should be treated with the same moral significance as hESCs as there is a grey area regarding their consciousness.
In conclusion, organoids and chimeric models raise many ethical and thought provoking questions, however, they offer invaluable research opportunities which could help many people, although they should be treated with more moral significance similar to that of hESCs.
References:
CAPPS B. Do Chimeras Have Minds?: The Ethics of Clinical Research on a Human–Animal Brain Model. Cambridge Quarterly of Healthcare Ethics. 2017;26(4):577-591. doi:10.1017/S0963180117000093
Grenier, K., Kao, J. & Diamandis, P. Three-dimensional modeling of human neurodegeneration: brain organoids coming of age. Mol Psychiatry 25, 254–274 (2020). https://doi.org/10.1038/s41380-019-0500-7
Kwisda, K., White, L. & Hübner, D. Ethical arguments concerning human-animal chimera research: a systematic review. BMC Med Ethics 21, 24 (2020). https://doi.org/10.1186/s12910-020-00465-7
Eigenhuis KN, Somsen HB, van der Kroeg M, Smeenk H, Korporaal AL, Kushner SA, de Vrij FMS, van den Berg DLC. A simplified protocol for the generation of cortical brain organoids. Front Cell Neurosci. 2023 Apr 4;17:1114420. doi: 10.3389/fncel.2023.1114420. PMID: 37082206; PMCID: PMC10110973.
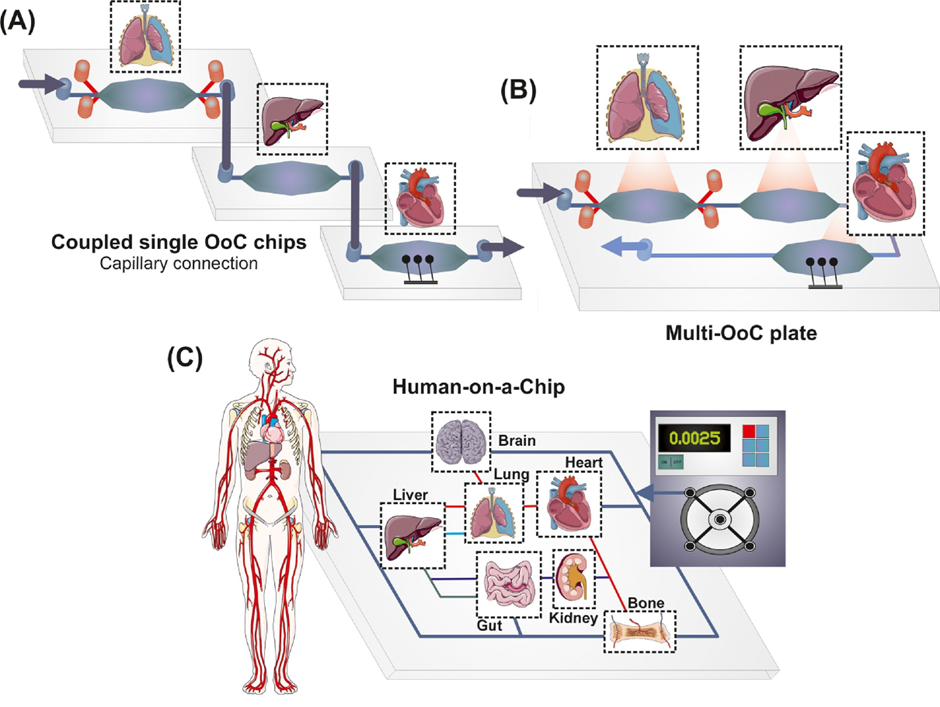


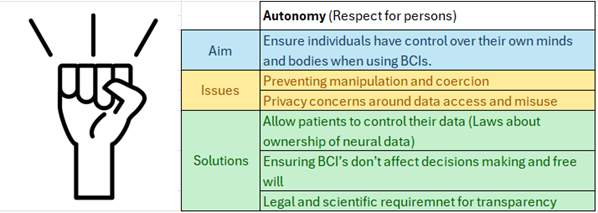
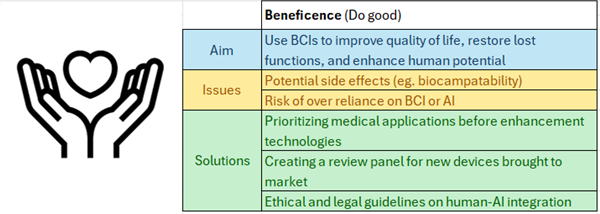
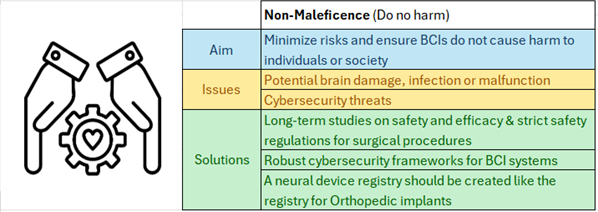





This is a good blog. It nicely demonstrates a good understanding of organ-on-a-chip technology and clearly explains its purpose and…
This is a good blog, very engaging with a good backgroud to 3D bioprinting. You could improve your blog with…
This is a good, very interesting blog about necrobotics. It explores the idea of necrobiotics which is fairly new approach…
This is a good blog. You introduce the reader to the topic of prosthetics and bionic limbs in a very…
This is a good blog introducing hernia mesh benefits and drawbacks. You create a narrative in this blog, which showcase…